Protein Domain Mimics as Modulators of Protein-Protein Interactions
- PMID: 28561588
- PMCID: PMC5615855
- DOI: 10.1021/acs.accounts.7b00130
Protein Domain Mimics as Modulators of Protein-Protein Interactions
Abstract
Protein-protein interactions (PPIs) are ubiquitous in biological systems and often misregulated in disease. As such, specific PPI modulators are desirable to unravel complex PPI pathways and expand the number of druggable targets available for therapeutic intervention. However, the large size and relative flatness of PPI interfaces make them challenging molecular targets. This Account describes our systematic approach using secondary and tertiary protein domain mimics (PDMs) to specifically modulate PPIs. Our strategy focuses on mimicry of regular secondary and tertiary structure elements from one of the PPI partners to inspire rational PDM design. We have compiled three databases (HIPPDB, SIPPDB, and DIPPDB) of secondary and tertiary structures at PPI interfaces to guide our designs and better understand the energetics of PPI secondary and tertiary structures. Our efforts have focused on three of the most common secondary and tertiary structures: α-helices, β-strands, and helix dimers (e.g., coiled coils). To mimic α-helices, we designed the hydrogen bond surrogate (HBS) as an isosteric PDM and the oligooxopiperazine helix mimetic (OHM) as a topographical PDM. The nucleus of the HBS approach is a peptide macrocycle in which the N-terminal i, i + 4 main-chain hydrogen bond is replaced with a covalent carbon-carbon bond. In mimicking a main-chain hydrogen bond, the HBS approach stabilizes the α-helical conformation while leaving all helical faces available for functionalization to tune binding affinity and specificity. The OHM approach, in contrast, envisions a tetrapeptide to mimic one face of a two-turn helix. We anticipated that placement of ethylene bridges between adjacent amides constrains the tetrapeptide backbone to mimic the i, i + 4, and i + 7 side chains on one face of an α-helix. For β-strands, we developed triazolamers, a topographical PDM where the peptide bonds are replaced by triazoles. The triazoles simultaneously stabilize the extended, zigzag conformation of β-strands and transform an otherwise ideal protease substrate into a stable molecule by replacement of the peptide bonds. We turned to a salt bridge surrogate (SBS) approach as a means for stabilizing very short helix dimers. As with the HBS approach, the SBS strategy replaces a noncovalent interaction with a covalent bond. Specifically, we used a bis-triazole linkage that mimics a salt bridge interaction to drive helix association and folding. Using this approach, we were able to stabilize helix dimers that are less than half of the length required to form a coiled coil from two independent strands. In addition to demonstrating the stabilization of desired structures, we have also shown that our designed PDMs specifically modulate target PPIs in vitro and in vivo. Examples of PPIs successfully targeted include HIF1α/p300, p53/MDM2, Bcl-xL/Bak, Ras/Sos, and HIV gp41. The PPI databases and designed PDMs created in these studies will aid development of a versatile set of molecules to probe complex PPI functions and, potentially, PPI-based therapeutics.
Conflict of interest statement
The authors declare the following competing financial interest(s): P.S.A. is a co-inventor on patent applications on secondary and tertiary structure mimics described in this paper. He is also cofounder of Inthera Bioscience, which is pursuing therapeutic applications of HBS and OHM technologies.
Figures





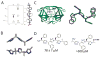

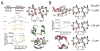
References
-
- Milroy LG, Grossmann TN, Hennig S, Brunsveld L, Ottmann C. Modulators of protein-protein interactions. Chem Rev. 2014;114:4695–4748. - PubMed
-
- Guharoy M, Chakrabarti P. Secondary structure based analysis and classification of biological interfaces: identification of binding motifs in protein-protein interactions. Bioinformatics. 2007;23:1909–1918. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

