Evolving Treatment Paradigm in Metastatic Renal Cell Carcinoma
- PMID: 28561652
- PMCID: PMC6855178
- DOI: 10.1200/EDBK_174469
Evolving Treatment Paradigm in Metastatic Renal Cell Carcinoma
Abstract
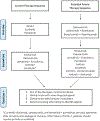
The treatment paradigm for advanced and metastatic renal cell carcinoma (mRCC) has evolved rapidly since the arrival of targeted therapies and novel immunotherapies. mRCC was previously treated only with cytokines. However, discoveries of mutations affecting the von Hippel-Lindau tumor suppressor gene (leading to increased expression of VEGF and hypoxia inducible factor/HIF-1) and of deregulations in the phosphatidylinositol-3 kinase/AKT/mTOR pathway (resulting in tumor angiogenesis, cell proliferation, and tumor growth) have led to the development of numerous targeted therapies. The U.S. Food and Drug Administration (FDA) has thus approved a total of nine targeted therapies since 2005, including VEGF tyrosine kinase inhibitors (sunitinib, pazopanib, axitinib, sorafenib, and lenvatinib), a monoclonal antibody targeting VEGF (bevacizumab), mTOR inhibitors (temsirolimus and everolimus), and a multityrosine kinase inhibitor (cabozantinib). Furthermore, the development of immune checkpoint inhibitors has again shifted the mRCC therapeutic landscape with the FDA's approval of nivolumab. Herein, we discuss the unprecedented changes in the field of clear cell histology mRCC in both the first-line and salvage settings, and we also discuss future therapies and recommend a treatment paradigm on sequencing of these agents.
Conflict of interest statement
Disclosures of potential conflicts of interest provided by the authors are available with the online article at
Figures


References
-
- Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. - PubMed
-
- RI Fisher, Rosenberg SA, Fyfe G Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6 (Suppl 1):S55–S57. - PubMed
-
- Clark JI, Wong MK, Kaufman HL et al. Impact of sequencing targeted therapies with high-dose interleukin-2 immunotherapy: an analysis of outcome and survival of patients with metastatic renal cell carcinoma from an on-going observational IL-2 clinical trial: PROCLAIM(SM). Clin Genitourin Cancer. 2017; 15:31–41. e4. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

