New Paradigm for Translational Modeling to Predict Long-term Tuberculosis Treatment Response
- PMID: 28561946
- PMCID: PMC5593171
- DOI: 10.1111/cts.12472
New Paradigm for Translational Modeling to Predict Long-term Tuberculosis Treatment Response
Abstract
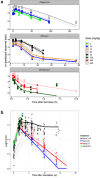
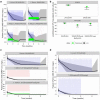
Disappointing results of recent tuberculosis chemotherapy trials suggest that knowledge gained from preclinical investigations was not utilized to maximal effect. A mouse-to-human translational pharmacokinetics (PKs) - pharmacodynamics (PDs) model built on a rich mouse database may improve clinical trial outcome predictions. The model included Mycobacterium tuberculosis growth function in mice, adaptive immune response effect on bacterial growth, relationships among moxifloxacin, rifapentine, and rifampin concentrations accelerating bacterial death, clinical PK data, species-specific protein binding, drug-drug interactions, and patient-specific pathology. Simulations of recent trials testing 4-month regimens predicted 65% (95% confidence interval [CI], 55-74) relapse-free patients vs. 80% observed in the REMox-TB trial, and 79% (95% CI, 72-87) vs. 82% observed in the Rifaquin trial. Simulation of 6-month regimens predicted 97% (95% CI, 93-99) vs. 92% and 95% observed in 2RHZE/4RH control arms, and 100% predicted and observed in the 35 mg/kg rifampin arm of PanACEA MAMS. These results suggest that the model can inform regimen optimization and predict outcomes of ongoing trials.
© 2017 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures


References
-
- World Health Organization . Global tuberculosis report 2016. http://www.who.int/tb/publications/global_report/en/ (2016).
-
- Dorman, S.E. et al Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the tuberculosis trials consortium. J. Infect. Dis. 206, 1030–1040 (2012). - PubMed
-
- Weiner, M. et al Rifapentine pharmacokinetics and tolerability in children and adults treated once weekly with rifapentine and isoniazid for latent tuberculosis infection. J. Pediatric Infect. Dis. Soc. 3, 132–145 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

