PLK4 phosphorylation of CP110 is required for efficient centriole assembly
- PMID: 28562169
- PMCID: PMC5499911
- DOI: 10.1080/15384101.2017.1325555
PLK4 phosphorylation of CP110 is required for efficient centriole assembly
Abstract
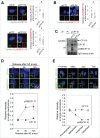
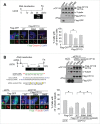
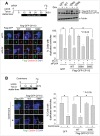
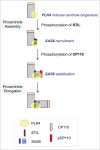
Centrioles are assembled during S phase and segregated into 2 daughter cells at the end of mitosis. The initiation of centriole assembly is regulated by polo-like kinase 4 (PLK4), the major serine/threonine kinase in centrioles. Despite its importance in centriole duplication, only a few substrates have been identified, and the detailed mechanism of PLK4 has not been fully elucidated. CP110 is a coiled-coil protein that plays roles in centriolar length control and ciliogenesis in mammals. Here, we revealed that PLK4 specifically phosphorylates CP110 at the S98 position. The phospho-resistant CP110 mutant inhibited centriole assembly, whereas the phospho-mimetic CP110 mutant induced centriole assembly, even in PLK4-limited conditions. This finding implies that PLK4 phosphorylation of CP110 is an essential step for centriole assembly. The phospho-mimetic form of CP110 augmented the centrosomal SAS6 level. Based on these results, we propose that the phosphorylated CP110 may be involved in the stabilization of cartwheel SAS6 during centriole assembly.
Keywords: CP110; PLK4; SAS6; centriole assembly; centrosome duplication.
Figures


Similar articles
-
Centriole distal-end proteins CP110 and Cep97 influence centriole cartwheel growth at the proximal end.J Cell Sci. 2022 Jul 15;135(14):jcs260015. doi: 10.1242/jcs.260015. Epub 2022 Jul 20. J Cell Sci. 2022. PMID: 35707992 Free PMC article.
-
Two-step phosphorylation of Ana2 by Plk4 is required for the sequential loading of Ana2 and Sas6 to initiate procentriole formation.Open Biol. 2017 Dec;7(12):170247. doi: 10.1098/rsob.170247. Open Biol. 2017. PMID: 29263250 Free PMC article.
-
Plk4 phosphorylates Ana2 to trigger Sas6 recruitment and procentriole formation.Curr Biol. 2014 Nov 3;24(21):2526-32. doi: 10.1016/j.cub.2014.08.061. Epub 2014 Sep 25. Curr Biol. 2014. PMID: 25264260 Free PMC article.
-
The PLK4-STIL-SAS-6 module at the core of centriole duplication.Biochem Soc Trans. 2016 Oct 15;44(5):1253-1263. doi: 10.1042/BST20160116. Biochem Soc Trans. 2016. PMID: 27911707 Free PMC article. Review.
-
Role of Polo-like Kinases Plk1 and Plk4 in the Initiation of Centriole Duplication-Impact on Cancer.Cells. 2022 Feb 24;11(5):786. doi: 10.3390/cells11050786. Cells. 2022. PMID: 35269408 Free PMC article. Review.
Cited by
-
Polo-Like Kinase 4's Critical Role in Cancer Development and Strategies for Plk4-Targeted Therapy.Front Oncol. 2021 Mar 12;11:587554. doi: 10.3389/fonc.2021.587554. eCollection 2021. Front Oncol. 2021. PMID: 33777739 Free PMC article. Review.
-
Asterless is a Polo-like kinase 4 substrate that both activates and inhibits kinase activity depending on its phosphorylation state.Mol Biol Cell. 2018 Nov 15;29(23):2874-2886. doi: 10.1091/mbc.E18-07-0445. Epub 2018 Sep 26. Mol Biol Cell. 2018. PMID: 30256714 Free PMC article.
-
Polo-like kinase 4 maintains centriolar satellite integrity by phosphorylation of centrosomal protein 131 (CEP131).J Biol Chem. 2019 Apr 19;294(16):6531-6549. doi: 10.1074/jbc.RA118.004867. Epub 2019 Feb 25. J Biol Chem. 2019. PMID: 30804208 Free PMC article.
-
The PLK4 inhibitor RP-1664 demonstrates potent single-agent efficacy in neuroblastoma models through a dual mechanism of sensitivity.Res Sq [Preprint]. 2025 Jul 29:rs.3.rs-7014295. doi: 10.21203/rs.3.rs-7014295/v1. Res Sq. 2025. PMID: 40766251 Free PMC article. Preprint.
-
PLK4-phosphorylated NEDD1 facilitates cartwheel assembly and centriole biogenesis initiations.J Cell Biol. 2021 Jan 4;220(1):e202002151. doi: 10.1083/jcb.202002151. J Cell Biol. 2021. PMID: 33351100 Free PMC article.
References
-
- Luders J, Stearns T. Microtubule-organizing centres: A re-evaluation. Nat Rev Mol Cell Biol 2007; 8(2):161-7; PMID:17245416; https://doi.org/10.1038/nrm2100 - DOI - PubMed
-
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet 2012; 13(3):189-203; PMID:22269907 - PubMed
-
- Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell 2007; 13(2):190-202; PMID:17681131; https://doi.org/10.1016/j.devcel.2007.07.002 - DOI - PubMed
-
- Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The polo kinase plk4 functions in centriole duplication. Nat Cell Biol 2005; 7(11):1140-6; PMID:16244668; https://doi.org/10.1038/ncb1320 - DOI - PubMed
-
- Bahtz R, Seidler J, Arnold M, Haselmann-Weiss U, Antony C, Lehmann WD, Hoffmann I. Gcp6 is a substrate of plk4 and required for centriole duplication. J Cell Sci 2012; 125(Pt 2):486-96; PMID:22302995; https://doi.org/10.1242/jcs.093930 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
