An Initiative to Improve the Quality of Care of Infants With Neonatal Abstinence Syndrome
- PMID: 28562267
- PMCID: PMC5470506
- DOI: 10.1542/peds.2016-3360
An Initiative to Improve the Quality of Care of Infants With Neonatal Abstinence Syndrome
Abstract
Background and objectives: The incidence of neonatal abstinence syndrome (NAS), a constellation of neurologic, gastrointestinal, and musculoskeletal disturbances associated with opioid withdrawal, has increased dramatically and is associated with long hospital stays. At our institution, the average length of stay (ALOS) for infants exposed to methadone in utero was 22.4 days before the start of our project. We aimed to reduce ALOS for infants with NAS by 50%.
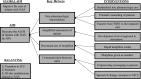
Methods: In 2010, a multidisciplinary team began several plan-do-study-act cycles at Yale New Haven Children's Hospital. Key interventions included standardization of nonpharmacologic care coupled with an empowering message to parents, development of a novel approach to assessment, administration of morphine on an as-needed basis, and transfer of infants directly to the inpatient unit, bypassing the NICU. The outcome measures included ALOS, morphine use, and hospital costs using statistical process control charts.
Results: There were 287 infants in our project, including 55 from the baseline period (January 2008 to February 2010) and 44 from the postimplementation period (May 2015 to June 2016). ALOS decreased from 22.4 to 5.9 days. Proportions of methadone-exposed infants treated with morphine decreased from 98% to 14%; costs decreased from $44 824 to $10 289. No infants were readmitted for treatment of NAS and no adverse events were reported.
Conclusions: Interventions focused on nonpharmacologic therapies and a simplified approach to assessment for infants exposed to methadone in utero led to both substantial and sustained decreases in ALOS, the proportion of infants treated with morphine, and hospital costs with no adverse events.
Copyright © 2017 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures


References
-
- Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e547 - PubMed
-
- Holmes AV, Atwood EC, Whalen B, et al. . Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics. 2016;137(6):e20152929. - PubMed
-
- Asti L, Magers JS, Keels E, Wispe J, McClead RE Jr. A quality improvement project to reduce length of stay for neonatal abstinence syndrome. Pediatrics. 2015;135(6). Available at: www.pediatrics.org/cgi/content/full/135/6/e1494 - PubMed
-
- Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP. Neonatal abstinence syndrome: assessment and management. Addict Dis. 1975;2(1–2):141–158 - PubMed
-
- Mehta A, Forbes KD, Kuppala VS. Neonatal abstinence syndrome management from prenatal counseling to postdischarge follow-up care: results of a national survey. Hosp Pediatr. 2013;3(4):317–323 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

