Maternal Use of Opioids During Pregnancy and Congenital Malformations: A Systematic Review
- PMID: 28562278
- PMCID: PMC5561453
- DOI: 10.1542/peds.2016-4131
Maternal Use of Opioids During Pregnancy and Congenital Malformations: A Systematic Review
Abstract
Context: Opioid use and abuse have increased dramatically in recent years, particularly among women.
Objectives: We conducted a systematic review to evaluate the association between prenatal opioid use and congenital malformations.
Data sources: We searched Medline and Embase for studies published from 1946 to 2016 and reviewed reference lists to identify additional relevant studies.
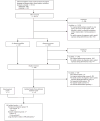
Study selection: We included studies that were full-text journal articles and reported the results of original epidemiologic research on prenatal opioid exposure and congenital malformations. We assessed study eligibility in multiple phases using a standardized, duplicate review process.
Data extraction: Data on study characteristics, opioid exposure, timing of exposure during pregnancy, congenital malformations (collectively or as individual subtypes), length of follow-up, and main findings were extracted from eligible studies.
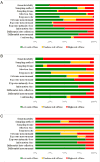
Results: Of the 68 studies that met our inclusion criteria, 46 had an unexposed comparison group; of those, 30 performed statistical tests to measure associations between maternal opioid use during pregnancy and congenital malformations. Seventeen of these (10 of 12 case-control and 7 of 18 cohort studies) documented statistically significant positive associations. Among the case-control studies, associations with oral clefts and ventricular septal defects/atrial septal defects were the most frequently reported specific malformations. Among the cohort studies, clubfoot was the most frequently reported specific malformation.
Limitations: Variabilities in study design, poor study quality, and weaknesses with outcome and exposure measurement.
Conclusions: Uncertainty remains regarding the teratogenicity of opioids; a careful assessment of risks and benefits is warranted when considering opioid treatment for women of reproductive age.
Copyright © 2017 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures
References
-
- National Institute on Drug Abuse; US Department of Health and Human Services. Research Report Series: Prescription Drug Abuse. Bethesda, MD: National Institute on Drug Abuse, US Department of Health and Human Services; 2014. (NIH Publication Number 15-4881).
-
- Paulozzi LJ, Mack KA, Hockenberry JM, Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC Vital signs: variation among states in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(26):563–568. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

