Guided self-organization and cortical plate formation in human brain organoids
- PMID: 28562594
- PMCID: PMC5824977
- DOI: 10.1038/nbt.3906
Guided self-organization and cortical plate formation in human brain organoids
Erratum in
-
Publisher Correction: Guided self-organization and cortical plate formation in human brain organoids.Nat Biotechnol. 2018 Oct 11;36(10):1016. doi: 10.1038/nbt1018-1016a. Nat Biotechnol. 2018. PMID: 30307926
Abstract
Three-dimensional cell culture models have either relied on the self-organizing properties of mammalian cells or used bioengineered constructs to arrange cells in an organ-like configuration. While self-organizing organoids excel at recapitulating early developmental events, bioengineered constructs reproducibly generate desired tissue architectures. Here, we combine these two approaches to reproducibly generate human forebrain tissue while maintaining its self-organizing capacity. We use poly(lactide-co-glycolide) copolymer (PLGA) fiber microfilaments as a floating scaffold to generate elongated embryoid bodies. Microfilament-engineered cerebral organoids (enCORs) display enhanced neuroectoderm formation and improved cortical development. Furthermore, reconstitution of the basement membrane leads to characteristic cortical tissue architecture, including formation of a polarized cortical plate and radial units. Thus, enCORs model the distinctive radial organization of the cerebral cortex and allow for the study of neuronal migration. Our data demonstrate that combining 3D cell culture with bioengineering can increase reproducibility and improve tissue architecture.
Conflict of interest statement
M.A.L. and J.A.K. have filed a patent application for use of this technology in future disease modeling and toxicology testing.
Figures
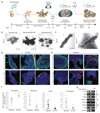
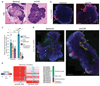
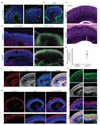
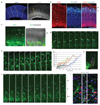
Comment in
-
A Little Bit of Guidance: Mini Brains on Their Route to Adolescence.Cell Stem Cell. 2017 Aug 3;21(2):157-158. doi: 10.1016/j.stem.2017.07.001. Cell Stem Cell. 2017. PMID: 28777940
References
-
- Rookmaaker MB, Schutgens F, Verhaar MC, Clevers H. Development and application of human adult stem or progenitor cell organoids. Nature Reviews Nephrology. 2015;11:546–554. - PubMed
-
- Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016;18:246–254. - PubMed
-
- Sasai Y. Next-Generation Regenerative Medicine: Organogenesis from Stem Cells in 3D Culture. Cell Stem Cell. 2013;12:520–530. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

