Efficacy and safety of neoadjuvant FOLFIRINOX for borderline resectable pancreatic adenocarcinoma: improved efficacy compared with gemcitabine-based regimen
- PMID: 28564637
- PMCID: PMC5542271
- DOI: 10.18632/oncotarget.17940
Efficacy and safety of neoadjuvant FOLFIRINOX for borderline resectable pancreatic adenocarcinoma: improved efficacy compared with gemcitabine-based regimen
Abstract
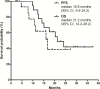
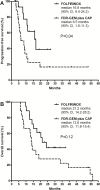
Borderline resectable pancreatic cancer (BRPC) is a potentially resectable disease but is associated with poorer survival compared to primary resectable disease. There has been no prospective trial that compare the efficacy of FOLFIRNOX and gemcitabine-based regimen for BRPC. Between February 2013 and December 2014, 18 patients with BRPC receiving FOLFIRINOX were reviewed retrospectively. For comparative analysis, data for all BRPC patients (n=18) in our previous phase 2 study of neoadjuvant fixed-dose rate-gemcitabine plus capecitabine were pooled. Patients received a median 6 cycles (range, 3-13) of FOLFIRINOX. Surgical resection was performed in 12 patients (67%) and R0 resection in 9 patients. Median progression-free survival (PFS) and overall survival (OS) were 16.8 (95% confidence interval [CI], 9.4-24.2) and 21.2 (95% CI, 14.2-28.2) months, respectively. Patients who underwent surgical resection showed significantly better PFS (p=0.01) and OS (p=0.003) than those unresected. In the exploratory analysis, patients receiving FOLFIRINOX showed significantly longer PFS compared to those receiving fixed-dose rate-gemcitabine plus capecitabine (median 16.8 months [95% CI, 9.4-24.2] vs. 6.5 months [1.6-11.3]; p = 0.04). There was a trend toward improved OS in patients who received FOLFIRINOX (median 21.2 months [95% CI, 14.2-28.2]) compared to those who received fixed-dose rate-gemcitabine plus capecitabine (13.6 months [11.8-15.4]; p=0.12). FOLFIRINOX was feasible and effective as neoadjuvant chemotherapy for patients with BRPC and may have improved efficacy compared to a gemcitabine-based regimen.
Keywords: FOLFIRINOX; chemotherapy; neoadjuvant; pancreatic cancer.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol. 2015;33:1770–1778. - PubMed
-
- Katz MH, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, Kindler HL, Alberts SR, Philip P, Lowy AM, Pisters PW, Posner MC, Berlin JD, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–2795. - PMC - PubMed
-
- National Comprehensive Cancer Network Pancreatic Adenocarcinoma (Version 1) assessed at September 26, 2016 2016.
-
- Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical