Myocardial fibrosis in congenital and pediatric heart disease
- PMID: 28565750
- PMCID: PMC5443200
- DOI: 10.3892/etm.2017.4224
Myocardial fibrosis in congenital and pediatric heart disease
Abstract
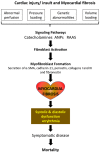
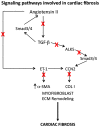
Cardiac fibrosis is a common phenomenon in different types of heart diseases, such as ischemic heart disease, inherited cardiomyopathy mutations, diabetes, and ageing and is associated with morbidity and mortality. Increased accumulation of extracellular matrix (ECM) that impacts cardiac function, is the underlying cause of fibrotic heart disease. There are four different types of cardiac fibrosis, including, reactive interstitial fibrosis, replacement fibrosis, infiltrative interstitial fibrosis and endomyocardial fibrosis. They are involved in the activation and transformation of cardiac fibroblasts to myofibroblasts, which participate in ECM production and fibrotic process and several inflammatory pathways. Besides the ECM proteins, myofibroblasts also express smooth muscle α-actin, SM22 and caldesmon and other markers related to fibrotic process. Most commonly employed techniques to assess myocardial fibrosis include stress echocardiography, cardiac magnetic resonance imaging and positron emission tomography. Because of the involvement of renin-angiotensin-II-aldosterone system, transforming growth factor-β signaling and activin-linked kinase 5 in the mechanisms of cardiac fibrosis, these pathways and the involved proteins are useful as therapeutic targets. However, because of the importance of these pathways in many other physiological functions, their therapeutic targeting needs to be approached with caution.
Keywords: cardiac fibrosis; extracellular matrix; inherited cardiomyopathy.
Figures
References
-
- Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216–225. doi: 10.1161/CIRCULATIONAHA.105.583500. - DOI - PubMed
-
- Farah MC, Castro CR, Moreira VM, Binotto MA, Guerra VC, Riso AA, Marcial MB, Lopes AA, Mathias W, Jr, Aiello VD. The impact of preexisting myocardial remodeling on ventricular function early after tetralogy of Fallot repair. J Am Soc Echocardiogr. 2010;23:912–918. doi: 10.1016/j.echo.2010.06.008. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
