Evaluation of the maintenance of stemness, viability, and differentiation potential of gingiva-derived stem-cell spheroids
- PMID: 28565764
- PMCID: PMC5443272
- DOI: 10.3892/etm.2017.4194
Evaluation of the maintenance of stemness, viability, and differentiation potential of gingiva-derived stem-cell spheroids
Abstract
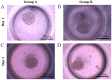
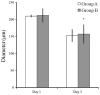
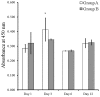
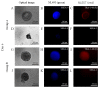
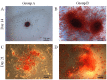
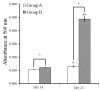
Gingiva-derived stem cells have been applied for tissue-engineering purposes and may be considered a favorable source of mesenchymal stem cells as harvesting stem cells from the mandible or maxilla may be performed with ease under local anesthesia. The present study was performed to fabricate stem-cell spheroids using concave microwells and to evaluate the maintenance of stemness, viability, and differentiation potential. Gingiva-derived stem cells were isolated, and the stem cells of 4×105 (group A) or 8×105 (group B) cells were seeded into polydimethylsiloxane-based, concave micromolds with 600 µm diameters. The morphology of the microspheres and the change of the diameters of the spheroids were evaluated. The viability of spheroids was qualitatively analyzed via Live/Dead kit assay. A cell viability analysis was performed on days 1, 3, 6, and 12 with Cell Counting Kit-8. The maintenance of stemness was evaluated with immunocytochemical staining using SSEA-4, TRA-1-60(R) (positive markers), and SSEA-1 (negative marker). Osteogenic, adipogenic, and chondrogenic differentiation potential was evaluated by incubating spheroids in osteogenic, adipogenic and chondrogenic induction medium, respectively. The gingiva-derived stem cells formed spheroids in the concave microwells. The diameters of the spheroids were larger in group A than in group B. The majority of cells in the spheroids emitted green fluorescence, indicating the presence of live cells at day 6. At day 12, the majority of cells in the spheroids emitted green fluorescence, and a small portion of red fluorescence was also noted, which indicated the presence of dead cells. The spheroids were positive for the stem-cell markers SSEA-4 and TRA-1-60(R) and were negative for SSEA-1, suggesting that these spheroids primarily contained undifferentiated human stem cells. Osteogenic, adipogenic, and chondrogenic differentiation was more evident with an increase of incubation time: Mineralized extracellular deposits were observed following Alizarin Red S staining at days 14 and 21; oil globules were increased at day 18 when compared with day 6; and Alcian blue staining was more evident at day 18 when compared with day 6. Within the limits of this study, stem-cell spheroids from gingival cells maintained the stemness, viability, and differentiation potential during the experimental periods. This method may be applied for a promising strategy for stem-cell therapy.
Keywords: cell culture techniques; cell survival; cellular spheroids; stem cell research; stem cells.
Figures



Similar articles
-
Osteogenic potential of cell spheroids composed of varying ratios of gingiva-derived and bone marrow stem cells using concave microwells.Exp Ther Med. 2018 Sep;16(3):2287-2294. doi: 10.3892/etm.2018.6462. Epub 2018 Jul 18. Exp Ther Med. 2018. PMID: 30186469 Free PMC article.
-
Evaluation of the shape, viability, stemness and osteogenic differentiation of cell spheroids formed from human gingiva-derived stem cells and osteoprecursor cells.Exp Ther Med. 2017 Jun;13(6):3467-3473. doi: 10.3892/etm.2017.4388. Epub 2017 Apr 26. Exp Ther Med. 2017. PMID: 28587426 Free PMC article.
-
Formation of size-controllable spheroids using gingiva-derived stem cells and concave microwells: Morphology and viability tests.Biomed Rep. 2016 Jan;4(1):97-101. doi: 10.3892/br.2015.539. Epub 2015 Nov 5. Biomed Rep. 2016. PMID: 26870343 Free PMC article.
-
Lovastatin increases the proliferation and osteoblastic differentiation of human gingiva-derived stem cells in three-dimensional cultures.Exp Ther Med. 2019 Nov;18(5):3425-3430. doi: 10.3892/etm.2019.7971. Epub 2019 Sep 5. Exp Ther Med. 2019. PMID: 31602217 Free PMC article.
-
Evaluation of fibroblast growth factor-2 on the proliferation of osteogenic potential and protein expression of stem cell spheroids composed of stem cells derived from bone marrow.Exp Ther Med. 2019 Jul;18(1):326-331. doi: 10.3892/etm.2019.7543. Epub 2019 May 3. Exp Ther Med. 2019. PMID: 31258669 Free PMC article.
Cited by
-
Vitamin D Enhanced the Osteogenic Differentiation of Cell Spheroids Composed of Bone Marrow Stem Cells.Medicina (Kaunas). 2021 Nov 19;57(11):1271. doi: 10.3390/medicina57111271. Medicina (Kaunas). 2021. PMID: 34833489 Free PMC article.
-
Fibroblast growth factor-4 maintains cellular viability while enhancing osteogenic differentiation of stem cell spheroids in part by regulating RUNX2 and BGLAP expression.Exp Ther Med. 2020 Sep;20(3):2013-2020. doi: 10.3892/etm.2020.8951. Epub 2020 Jun 26. Exp Ther Med. 2020. PMID: 32782511 Free PMC article.
-
Osteogenic potential of cell spheroids composed of varying ratios of gingiva-derived and bone marrow stem cells using concave microwells.Exp Ther Med. 2018 Sep;16(3):2287-2294. doi: 10.3892/etm.2018.6462. Epub 2018 Jul 18. Exp Ther Med. 2018. PMID: 30186469 Free PMC article.
-
Morphological stability, cellular viability and stem cell marker expression of three-dimensional cultures of stem cells from bone marrow and periodontium.Biomed Rep. 2021 Jan;14(1):9. doi: 10.3892/br.2020.1385. Epub 2020 Nov 11. Biomed Rep. 2021. PMID: 33235724 Free PMC article.
-
The effects of doxorubicin-loaded liposomes on viability, stem cell surface marker expression and secretion of vascular endothelial growth factor of three-dimensional stem cell spheroids.Exp Ther Med. 2018 Jun;15(6):4950-4960. doi: 10.3892/etm.2018.6064. Epub 2018 Apr 13. Exp Ther Med. 2018. PMID: 29805519 Free PMC article.
References
-
- Park JB, Bae SS, Lee PW, Lee W, Park YH, Kim H, Lee K, Kim I. Comparison of stem cells derived from periosteum and bone marrow of jaw bone and long bone in rabbit models. Tissue Eng Regen Med. 2012;9:224–230. doi: 10.1007/s13770-012-0343-7. - DOI
-
- Park JB, Lee KS, Lee W, Kim HS, Lee KH, Kim IS. Establishment of the chronic bone defect model in experimental model mandible and evaluation of the efficacy of the mesenchymal stem cells in enhancing bone regeneration. Tissue Eng Regen Med. 2013;10:18–24. doi: 10.1007/s13770-013-0368-6. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
