Comparison of the purity and vitality of natural killer cells with different isolation kits
- PMID: 28565780
- PMCID: PMC5443303
- DOI: 10.3892/etm.2017.4189
Comparison of the purity and vitality of natural killer cells with different isolation kits
Abstract
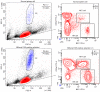
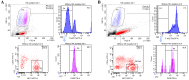
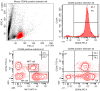
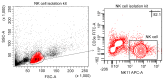
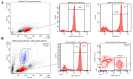
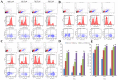
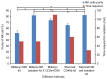
Natural killer (NK) cells are innate lymphocytes that aid in the protection of the host from infectious diseases and cancer. In vitro studies of NK cells have provided a foundation for developing clinical adoptive NK-cell transferred immunotherapy against human tumors. To elucidate the functions and mechanisms of NK cell populations, it is important to develop an optimal, highly reproducible and reliable isolation method. The present comparative study was performed with four different NK cell isolation kits of magnetic bead labeling made by Miltenyi and Stemcell companies, including positive selection kits [cluster of differentiation (CD)-49b, using the monoclonal antibody DX5) MicroBeads] and negative selection kits. In addition, the viability of NK cells isinterleukin-2 (IL-2)-dependent in vitro and thus the concentration of IL-2 is critical for maintaining longer cell viability of NK cells. NK cell purity and viability after culturing, for 24, 48 or 72 h, with or without IL-2 (0, 100, 300 or 500 U/ml) was investigated in the present study. Purity of NK cells varied depending on the purification kit used, despite the same method being applied. Furthermore, more granulocytes were present in purified NK cells using Miltenyi sorting kits, particularly when using the negative selection kit. The main disadvantage of DX5-positive selection using the Stemcell and Miltenyi kits was that a high percentage of CD3ε+ cells were mixed into the isolated NK cells. Additionally, a significant difference of NK cell purity (P=0.003) was observed while purification was performed using different surface markers. As a consequence, the use of the positive selection kit was modified and subsequently a significantly higher purity (P=0.002) and yield (P=0.004) of NK cells was obtained. Moreover, the purity of NK cells and viability with or without a range of concentrations of IL-2 was compared. Results indicated that with a higher IL-2 concentration, the NK cell purity and viability were significantly higher (P<0.05). To our knowledge, this is the first report that has compared the disadvantages of four commercial NK cell isolation kits from two well-known companies, and identified the effect of NK cell purity and viability, using different concentrations of IL-2. To conclude, the results of the present study are fundamental in aiding the further development of NK cell therapy protocols for murine in vivo models.
Keywords: DX5; interleukin-2; magnetic-activated cell sorting; natural killer cell culture; natural killer cell isolation; natural killer cells.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
