Gambogenic acid induces cell growth inhibition, cell cycle arrest and metastasis inhibition in choroidal melanoma in a dose-dependent manner
- PMID: 28565863
- PMCID: PMC5443308
- DOI: 10.3892/etm.2017.4252
Gambogenic acid induces cell growth inhibition, cell cycle arrest and metastasis inhibition in choroidal melanoma in a dose-dependent manner
Abstract
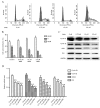
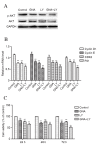
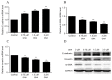
The aim of the present study was to explore the effects of gambogenic acid (GNA) on the malignant behaviors of choroidal melanoma cells, including cell viability, cell cycle, migration and invasion, and to elucidate the underlying regulatory mechanism. The human choroidal melanoma cell line OCM-1 was treated with different concentrations of GNA and cell viability, colony formation ability, cell cycle, migration and invasion were analyzed. Additionally, cells were incubated with or without LY294002, a specific inhibitor of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, for 24 h. Levels of cell cycle-associated proteins (cyclin D1, cyclin E, cyclin-dependent kinase 2 and P21), epithelial-mesenchymal transition (EMT)-associated molecules (epithelial-cadherin, α-smooth muscle actin and vimentin) and phosphorylated (p)-AKT/AKT were determined using reverse transcription-quantitative polymerase chain reaction and western blot analysis. The results demonstrated that GNA significantly inhibited cell viability and induced cell cycle arrest at the G0/G1 phase in a dose-dependent manner (P<0.01). Furthermore, GNA administration significantly suppressed cell migration and invasion in a dose-dependent manner (P<0.01). Treatment with GNA or LY294002 induced a marked decrease in the expression of p-AKT/AKT, a significant downregulation in cell cycle-associated molecules (P<0.01), and a significant decrease in cell viability (P<0.01). Co-treatment with LY294002 and GNA had an additive effect on the growth of OCM-1 cells. In conclusion, the results of the present study suggest that treatment with GNA may inhibit cell viability and induce G0/G1 arrest. Furthermore, GNA may also inhibit cell metastasis via regulating EMT-associated molecules. The PI3K/Akt signaling pathway may be a key mechanism involved in the progression of choroidal melanoma, and GNA may serve as a potential therapeutic reagent for the treatment of this disease.
Keywords: cell cycle arrest; choroidal melanoma; gambogenic acid; growth; metastasis.
Figures
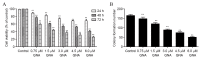

Similar articles
-
Gambogenic Acid Suppresses Malignant Progression of Non-Small Cell Lung Cancer via GCH1-Mediated Ferroptosis.Pharmaceuticals (Basel). 2025 Mar 6;18(3):374. doi: 10.3390/ph18030374. Pharmaceuticals (Basel). 2025. PMID: 40143150 Free PMC article.
-
[Study of gambogenic acid-induced apoptosis of melanoma B16 cells through PI3K/Akt/mTOR signaling pathways].Zhongguo Zhong Yao Za Zhi. 2014 May;39(9):1666-9. Zhongguo Zhong Yao Za Zhi. 2014. PMID: 25095381 Chinese.
-
Gambogenic acid induces ferroptosis in melanoma cells undergoing epithelial-to-mesenchymal transition.Toxicol Appl Pharmacol. 2020 Aug 15;401:115110. doi: 10.1016/j.taap.2020.115110. Epub 2020 Jun 10. Toxicol Appl Pharmacol. 2020. PMID: 32533954
-
Blocking PI3K/Akt signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition.Oncol Rep. 2014 Aug;32(2):559-66. doi: 10.3892/or.2014.3220. Epub 2014 May 29. Oncol Rep. 2014. PMID: 24889918
-
Equol inhibits proliferation of human gastric carcinoma cells via modulating Akt pathway.World J Gastroenterol. 2015 Sep 28;21(36):10385-99. doi: 10.3748/wjg.v21.i36.10385. World J Gastroenterol. 2015. PMID: 26420965 Free PMC article.
Cited by
-
Oridonin inhibits migration, invasion, adhesion and TGF-β1-induced epithelial-mesenchymal transition of melanoma cells by inhibiting the activity of PI3K/Akt/GSK-3β signaling pathway.Oncol Lett. 2018 Jan;15(1):1362-1372. doi: 10.3892/ol.2017.7421. Epub 2017 Nov 15. Oncol Lett. 2018. PMID: 29399187 Free PMC article.
-
Degradation strategy of cyclin D1 in cancer cells and the potential clinical application.Front Oncol. 2022 Aug 18;12:949688. doi: 10.3389/fonc.2022.949688. eCollection 2022. Front Oncol. 2022. PMID: 36059670 Free PMC article. Review.
-
Gambogenic acid inhibits the proliferation of small‑cell lung cancer cells by arresting the cell cycle and inducing apoptosis.Oncol Rep. 2019 Mar;41(3):1700-1706. doi: 10.3892/or.2018.6950. Epub 2018 Dec 21. Oncol Rep. 2019. PMID: 30592285 Free PMC article.
-
Gambogenic Acid Suppresses Malignant Progression of Non-Small Cell Lung Cancer via GCH1-Mediated Ferroptosis.Pharmaceuticals (Basel). 2025 Mar 6;18(3):374. doi: 10.3390/ph18030374. Pharmaceuticals (Basel). 2025. PMID: 40143150 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
