Enantioselective CuH-Catalyzed Hydroacylation Employing Unsaturated Carboxylic Acids as Aldehyde Surrogates
- PMID: 28565905
- PMCID: PMC5587210
- DOI: 10.1021/jacs.7b04937
Enantioselective CuH-Catalyzed Hydroacylation Employing Unsaturated Carboxylic Acids as Aldehyde Surrogates
Abstract
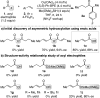
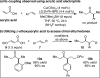
The direct asymmetric copper hydride (CuH)-catalyzed coupling of α,β-unsaturated carboxylic acids to aryl alkenes to access chiral α-aryl dialkyl ketones is reported. A variety of substrate substitution patterns, sensitive functional groups, and heterocycles are tolerated in this reaction, which significantly expands the range of accessible products compared with existing hydroacylation methodology. Although mechanistic studies are ongoing, we propose that CuH-catalyzed silylation of unsaturated acids occurs to access a uniquely effective acyl electrophilic coupling partner.
Figures


References
-
- Corey EJ, Kürti L. Enantioselective Chemical Synthesis: Methods, Logic and Practice. Direct Book Publishing; Dallas: 2010.
-
-
For general approaches toward quaternary α-aryl ketones, see reference . For reviews on and selected examples of catalytic asymmetric synthesis of quaternary α-aryl ketones: Bellina F, Rossi R. Chem. Rev. 2010;110:1082.Johansson CCC, Colacot TJ. Angew. Chem., Int. Ed. 2010;49:676.Bella M, Gasperi T. Synthesis. 2009;10:1583.Hamada T, Chieffi A, Åhman J, Buchwald SL. J. Am. Chem. Soc. 2002;124:1261.Lia X, Weng Z, Hartwig JF. J. Am. Chem. Soc. 2008;130:195.Trost BM, Schroeder GM, Kristensen J. Angew. Chem., Int. Ed. 2002;41:3492.Kano T, Hayashi Y, Maruoka K. J. Am. Chem. Soc. 2013;135:7134.Xie X, Chen Y, Ma D. J. Am. Chem. Soc. 2006;128:16050.
-
-
- Bordwell FG, Harrelson JA., Jr Can. J. Chem. 1990;68:1714.
-
-
For reviews and selected examples of asymmetric protonation reactions to access chiral α-aryl ketones, see: Oudeyer S, Briere J-F, Levacher V. Eur. J. Org. Chem. 2014:6103.Mohr JT, Hong AY, Stoltz BM. Nat. Chem. 2009;1:359.Cheon CH, Kanno O, Toste FD. J. Am. Chem. Soc. 2011;133:13248.Cheon CH, Yamamoto H. J. Am. Chem. Soc. 2008;130:9246.Yanagisawa A, Touge T, Arai T. Angew. Chem., Int. Ed. 2005;44:1546.
-
-
- Lundin PM, Esquivias J, Fu GC. Angew. Chem., Int. Ed. 2009;48:154. - PMC - PubMed
- Lou S, Fu GC. J. Am. Chem. Soc. 2010;132:1264. - PMC - PubMed
- Cherney AH, Kadunce NT, Reisman SE. J. Am. Chem. Soc. 2013;135:7442. - PubMed
- Oost R, Misale A, Maulide N. Angew. Chem., Int. Ed. 2016;55:4587. - PubMed
- Kang BC, Nam DG, Hwang G-S, Ryu DH. Org. Lett. 2015;17:4810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

