Colchicine Depolymerizes Microtubules, Increases Junctophilin-2, and Improves Right Ventricular Function in Experimental Pulmonary Arterial Hypertension
- PMID: 28566298
- PMCID: PMC5669202
- DOI: 10.1161/JAHA.117.006195
Colchicine Depolymerizes Microtubules, Increases Junctophilin-2, and Improves Right Ventricular Function in Experimental Pulmonary Arterial Hypertension
Abstract
Background: Pulmonary arterial hypertension (PAH) is a lethal disease characterized by obstructive pulmonary vascular remodeling and right ventricular (RV) dysfunction. Although RV function predicts outcomes in PAH, mechanisms of RV dysfunction are poorly understood, and RV-targeted therapies are lacking. We hypothesized that in PAH, abnormal microtubular structure in RV cardiomyocytes impairs RV function by reducing junctophilin-2 (JPH2) expression, resulting in t-tubule derangements. Conversely, we assessed whether colchicine, a microtubule-depolymerizing agent, could increase JPH2 expression and enhance RV function in monocrotaline-induced PAH.
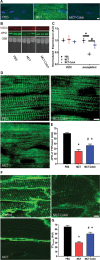
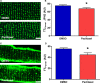
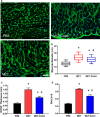
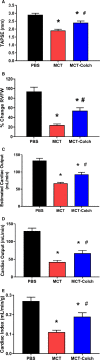
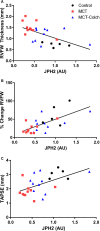
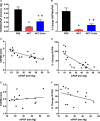
Methods and results: Immunoblots, confocal microscopy, echocardiography, cardiac catheterization, and treadmill testing were used to examine colchicine's (0.5 mg/kg 3 times/week) effects on pulmonary hemodynamics, RV function, and functional capacity. Rats were treated with saline (n=28) or colchicine (n=24) for 3 weeks, beginning 1 week after monocrotaline (60 mg/kg, subcutaneous). In the monocrotaline RV, but not the left ventricle, microtubule density is increased, and JPH2 expression is reduced, with loss of t-tubule localization and t-tubule disarray. Colchicine reduces microtubule density, increases JPH2 expression, and improves t-tubule morphology in RV cardiomyocytes. Colchicine therapy diminishes RV hypertrophy, improves RV function, and enhances RV-pulmonary artery coupling. Colchicine reduces small pulmonary arteriolar thickness and improves pulmonary hemodynamics. Finally, colchicine increases exercise capacity.
Conclusions: Monocrotaline-induced PAH causes RV-specific derangement of microtubules marked by reduction in JPH2 and t-tubule disarray. Colchicine reduces microtubule density, increases JPH2 expression, and improves both t-tubule architecture and RV function. Colchicine also reduces adverse pulmonary vascular remodeling. These results provide biological plausibility for a clinical trial to repurpose colchicine as a RV-directed therapy for PAH.
Keywords: T‐tubules; pulmonary hypertension; right ventricular failure; right ventricular pressure overload.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures


References
-
- Benza RL, Miller DP, Gomberg‐Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long‐Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122:164–172. - PubMed
-
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud‐Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. - PubMed
-
- Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

