The Superiority of Vonoprazan-based First-line Triple Therapy with Clarithromycin: A Prospective Multi-center Cohort Study on Helicobacter pylori Eradication
- PMID: 28566587
- PMCID: PMC5498188
- DOI: 10.2169/internalmedicine.56.7833
The Superiority of Vonoprazan-based First-line Triple Therapy with Clarithromycin: A Prospective Multi-center Cohort Study on Helicobacter pylori Eradication
Abstract
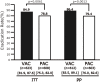
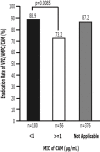
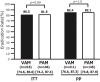
Objective We evaluated the safety and efficacy of vonoprazan-based amoxicillin and clarithromycin 7-day triple therapy (VAC) in comparison to proton pump inhibitor (PPI)-based (PAC) as a first-line treatment and vonoprazan-based amoxicillin and metronidazole 7-day triple therapy (VAM) in comparison to PPI-based (PAM) as a second-line treatment for the eradication of Helicobacter pylori in Japan. Methods We performed a non-randomized, multi-center, parallel-group study to compare first-line VAC to PAC and second-line VAM to PAM. A pre-planned subgroup analysis on CAM resistance was also performed. Safety was evaluated with an adverse effects questionnaire (AEQ), which was completed by patients during therapy. Results The first-line eradication rates (ER) in the intention-to-treat (ITT) and per protocol (PP) analyses were 84.9% (95% CI: 81.9-87.6%, n=623) and 86.4% (83.5-89.1%, n=612), respectively, for VAC and 78.8% (75.3-82.0%, n=608) and 79.4% (76.0-82.6%, n=603), respectively, for PAC. The ER of VAC was higher than that of PAC in the ITT (p=0.0061) and PP analyses (p=0.0013). The ERs for VAC in patients with CAM-resistant and CAM-susceptible bacteria were 73.2% (59.7-84.2%, n=56) and 88.9% (83.4-93.1%, n=180), respectively. PAC was associated with higher AEQ scores for diarrhea, nausea, headache, and general malaise. In the second-line ITT and PP analyses VAM achieved ERs of 80.5% (74.6-85.6%, n=216) and 82.4% (76.6-87.3%, n=211), respectively, while PAM achieved ERs of 81.5% (74.2-87.4%, n=146) and 82.1% (74.8-87.9%, n=145), respectively. No significant differences were observed in the ITT (p=0.89) or PP (p=1.0) analyses. Conclusion The ER of first-line VAC was higher than that of PAC, but still <90%. No difference was observed between second-line VAM and PAM. Vonoprazan-based triple therapy was safe and well tolerated.
Keywords: 7-day triple therapy; Helicobacter pylori; eradication; vonoprazan.
Figures
Similar articles
-
Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: A multicenter, prospective, randomized trial.Helicobacter. 2018 Apr;23(2):e12456. doi: 10.1111/hel.12456. Epub 2017 Dec 21. Helicobacter. 2018. PMID: 29271026 Clinical Trial.
-
Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for Helicobacter pylori eradication.Ann Clin Microbiol Antimicrob. 2018 Jun 28;17(1):29. doi: 10.1186/s12941-018-0281-x. Ann Clin Microbiol Antimicrob. 2018. PMID: 29950163 Free PMC article.
-
Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: A multicenter retrospective study in clinical practice.J Dig Dis. 2016 Oct;17(10):670-675. doi: 10.1111/1751-2980.12398. J Dig Dis. 2016. PMID: 27534444
-
Vonoprazan-based therapies versus PPI-based therapies in patients with H. pylori infection: Systematic review and meta-analyses of randomized controlled trials.Helicobacter. 2024 May-Jun;29(3):e13094. doi: 10.1111/hel.13094. Helicobacter. 2024. PMID: 38790090
-
Efficacy of Vonoprazan for Helicobacter pylori Eradication.Intern Med. 2020 Jan 15;59(2):153-161. doi: 10.2169/internalmedicine.2521-18. Epub 2019 Jun 27. Intern Med. 2020. PMID: 31243237 Free PMC article. Review.
Cited by
-
Vonoprazan-based triple therapy is effective for Helicobacter pylori eradication irrespective of clarithromycin susceptibility.J Gastroenterol. 2020 Nov;55(11):1054-1061. doi: 10.1007/s00535-020-01723-6. Epub 2020 Sep 15. J Gastroenterol. 2020. PMID: 32930864
-
Efficacy and safety of triple therapy containing berberine, amoxicillin, and vonoprazan for Helicobacter pylori initial treatment: A randomized controlled trial.Chin Med J (Engl). 2023 Jul 20;136(14):1690-1698. doi: 10.1097/CM9.0000000000002696. Epub 2023 May 22. Chin Med J (Engl). 2023. PMID: 37469024 Free PMC article. Clinical Trial.
-
Is a Potassium-Competitive Acid Blocker Truly Superior to Proton Pump Inhibitors in Terms of Helicobacter pylori Eradication?Gut Liver. 2021 Nov 15;15(6):799-810. doi: 10.5009/gnl20242. Gut Liver. 2021. PMID: 33850058 Free PMC article. Review.
-
Vonoprazan a novel potassium competitive acid blocker; another leap forward.Prz Gastroenterol. 2024;19(2):135-142. doi: 10.5114/pg.2024.139426. Epub 2024 May 8. Prz Gastroenterol. 2024. PMID: 38939071 Free PMC article.
-
Response to: Comment on "First-Line Helicobacter pylori Eradication with Vonoprazan, Clarithromycin, and Metronidazole in Patients Allergic to Penicillin".Gastroenterol Res Pract. 2018 Mar 22;2018:8046838. doi: 10.1155/2018/8046838. eCollection 2018. Gastroenterol Res Pract. 2018. PMID: 29767036 Free PMC article. No abstract available.
References
-
- Gonzalez CA, Megraud F, Buissonniere A, et al. . Helicobacter pylori infection assessed by ELISA and by immunoblot and noncardia gastric cancer risk in a prospective study: the Eurgast-EPIC project. Ann Oncol 23: 1320-1324, 2012. - PubMed
-
- Greenberg ER, Park JY. Effectiveness of Helicobacter pylori eradication. In: Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer IARC Working Group Report Volume 8. International Agency for Research on Cancer. Albert Thomas, Lyon Cedex, 2014: 64-71.
-
- Asaka M, Sugiyama T, Kato M, et al. . A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 6: 254-261, 2001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

