Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells
- PMID: 28566702
- PMCID: PMC5451421
- DOI: 10.1038/s41598-017-02890-y
Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells
Abstract
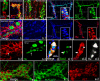
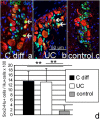
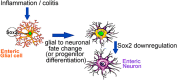
Mechanisms mediating adult enteric neurogenesis are largely unknown. Using inflammation-associated neurogenesis models and a transgenic approach, we aimed to understand the cell-source for new neurons in infectious and inflammatory colitis. Dextran sodium sulfate (DSS) and Citrobacter rodentium colitis (CC) was induced in adult mice and colonic neurons were quantified. Sox2GFP and PLP1GFP mice confirmed the cell-type specificity of these markers. Sox2CreER:YFP and PLP1creER:tdT mice were used to determine the fate of these cells after colitis. Sox2 expression was investigated in colonic neurons of human patients with Clostridium difficile or ulcerative colitis. Both DSS and CC led to increased colonic neurons. Following colitis in adult Sox2CreER:YFP mice, YFP initially expressed predominantly by glia becomes expressed by neurons following colitis, without observable DNA replication. Similarly in PLP1CreER:tdT mice, PLP1 cells that co-express S100b but not RET also give rise to neurons following colitis. In human colitis, Sox2-expressing neurons increase from 1-2% to an average 14% in colitis. The new neurons predominantly express calretinin, thus appear to be excitatory. These results suggest that colitis promotes rapid enteric neurogenesis in adult mice and humans through differentiation of Sox2- and PLP1-expressing cells, which represent enteric glia and/or neural progenitors. Further defining neurogenesis will improve understanding and treatment of injury-associated intestinal motility/sensory disorders.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
-
- Srinivasan, S. & Wiley, J. W. New insights into neural injury, repair, and adaptation in visceral afferents and the enteric nervous system. Curr Opin Gastroenterol16, 78–82, doi:00001574-200001000-00014 [pii] (2000). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

