Potent anti-cancer effects of less polar Curcumin analogues on gastric adenocarcinoma and esophageal squamous cell carcinoma cells
- PMID: 28566729
- PMCID: PMC5451386
- DOI: 10.1038/s41598-017-02666-4
Potent anti-cancer effects of less polar Curcumin analogues on gastric adenocarcinoma and esophageal squamous cell carcinoma cells
Abstract
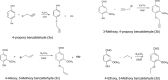
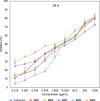
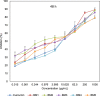
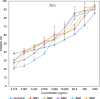
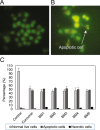
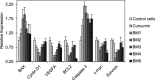
Curcumin and its chalcone derivatives inhibit the growth of human cancer cells. It is reported that replacement of two OH groups in curcumin with less polar groups like methoxy increases its anti-proliferative activity. In this study, we explored benzylidine cyclohexanone derivatives with non-polar groups, to see if they possess increased anti-cancer activity. Novel 2,6-bis benzylidine cyclohexanone analogues of curcumin were synthesized, and their inhibitory effects on gastric adenocarcinoma (AGS) and esophageal squamous cell carcinoma (KYSE30) cancer cells were studied using an MTT assay. Cell apoptosis was detected by EB/AO staining, and cell cycle was analyzed by flow cytometry. Real-time PCR was performed for gene expression analysis. All synthesized analogues were cytotoxic toward gastric and esophageal cancer cells and showed lower IC50 values than curcumin. Treatment with 2,6-Bis-(3-methoxy-4-propoxy-benzylidene)-cyclohexanone (BM2) was 17 times more toxic than curcumin after 48 h incubation. All novel compounds were more effective than curcumin in apoptosis induction and cell cycle arrest at G1 phase. These results suggest that less polar analogues of curcumin have potent cytotoxicity in vitro. However, they need to be investigated further, especially with animal tumor models, to confirm their chemotherapeutic activity in vivo.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Kocaadam, B. & Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Critical reviews in food science and nutrition, 00-00 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

