Relative Contribution of Prolyl Hydroxylase-Dependent and -Independent Degradation of HIF-1alpha by Proteasomal Pathways in Cerebral Ischemia
- PMID: 28566998
- PMCID: PMC5434458
- DOI: 10.3389/fnins.2017.00239
Relative Contribution of Prolyl Hydroxylase-Dependent and -Independent Degradation of HIF-1alpha by Proteasomal Pathways in Cerebral Ischemia
Abstract
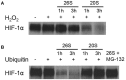
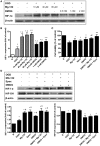
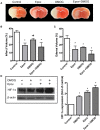
Hypoxia inducible factor-1 (HIF-1) is a key regulator in hypoxia and can determine the fate of brain cells during ischemia. However, the mechanism of HIF-1 regulation is still not fully understood in ischemic brains. We tested a hypothesis that both the 26S and the 20S proteasomal pathways were involved in HIF-1α degradation under ischemic conditions. Using in vitro ischemic model (oxygen and glucose deprivation) and a mouse model of middle cerebral artery occlusion, we tested effects of inhibitors of proteasomes and prolyl hydroxylase (PHD) on HIF-1α stability and brain injury in cerebral ischemia. We observed that 30 and 60 min of oxygen-glucose deprivation significantly increased the 20S proteasomal activity. We demonstrated that proteasome inhibitors increased HIF-1α stabilization and cell viability and were more effective than PHD inhibitors in primary cultured cortical neurons exposed to oxygen and glucose deprivation. Furthermore, the administration of the proteasome inhibitor, epoxomicin, to mice resulted in smaller infarct size and brain edema than a PHD inhibitor. Our results indicate that 20S proteasomes are involved in HIF-1α degradation in ischemic neurons and that proteasomal inhibition provides more HIF-1α stabilization and neuroprotection than PHD inhibition in cerebral ischemia.
Keywords: HIF-1; neurons; oxidative stress; proteasome; stroke.
Figures

References
-
- Baranova O., Miranda L. F., Pichiule P., Dragatsis I., Johnson R. S., Chavez J. C. (2007). Neuron-specific inactivation of the hypoxia inducible factor 1 increases brain injury in a mouse model of transient focal cerebral ischemia. J. Neurosci. 27:6320. 10.1523/JNEUROSCI.0449-07.2007 - DOI - PMC - PubMed
-
- Berti R., Williams A. J., Velarde L. C., Moffett J. R., Elliott P. J., Adams J., et al. . (2003). Effect of the proteasome inhibitor MLN519 on the expression of inflammatory molecules following middle cerebral artery occlusion and reperfusion in the rat. Neurotox. Res. 5, 505–514. 10.1007/BF03033160 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

