Nasal IgA Provides Protection against Human Influenza Challenge in Volunteers with Low Serum Influenza Antibody Titre
- PMID: 28567036
- PMCID: PMC5434144
- DOI: 10.3389/fmicb.2017.00900
Nasal IgA Provides Protection against Human Influenza Challenge in Volunteers with Low Serum Influenza Antibody Titre
Abstract
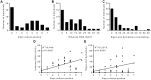
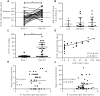
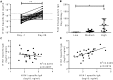
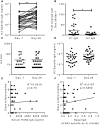
In spite of there being a number of vaccines, influenza remains a significant global cause of morbidity and mortality. Understanding more about natural and vaccine induced immune protection against influenza infection would help to develop better vaccines. Virus specific IgG is a known correlate of protection, but other factors may help to reduce viral load or disease severity, for example IgA. In the current study we measured influenza specific responses in a controlled human infection model using influenza A/California/2009 (H1N1) as the challenge agent. Volunteers were pre-selected with low haemagglutination inhibition (HAI) titres in order to ensure a higher proportion of infection; this allowed us to explore the role of other immune correlates. In spite of HAI being uniformly low, there were variable levels of H1N1 specific IgG and IgA prior to infection. There was also a range of disease severity in volunteers allowing us to compare whether differences in systemic and local H1N1 specific IgG and IgA prior to infection affected disease outcome. H1N1 specific IgG level before challenge did not correlate with protection, probably due to the pre-screening for individuals with low HAI. However, the length of time infectious virus was recovered from the nose was reduced in patients with higher pre-existing H1N1 influenza specific nasal IgA or serum IgA. Therefore, IgA contributes to protection against influenza and should be targeted in vaccines.
Keywords: Human Infection Challenge study; IgA; influenza; nasal; vaccine.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

