Research Ethics Governance in Times of Ebola
- PMID: 28567113
- PMCID: PMC5444563
- DOI: 10.1093/phe/phw039
Research Ethics Governance in Times of Ebola
Abstract
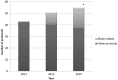
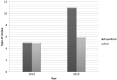
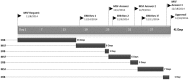
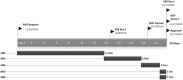
The Médecins Sans Frontières (MSF) ethics review board (ERB) has been solicited in an unprecedented way to provide advice and review research protocols in an 'emergency' mode during the recent Ebola epidemic. Twenty-seven Ebola-related study protocols were reviewed between March 2014 and August 2015, ranging from epidemiological research, to behavioural research, infectivity studies and clinical trials with investigational products at (very) early development stages. This article examines the MSF ERB's experience addressing issues related to both the process of review and substantive ethical issues in this context. These topics include lack of policies regarding blood sample collection and use, and engaging communities regarding their storage and future use; exclusion of pregnant women from clinical and vaccine trials; and the difficulty of implementing timely and high-quality qualitative/anthropological research to consider potential upfront harms. Having noticed different standards across ethics committees (ECs), we propose that when multiple ethics reviews of clinical and vaccine trials are carried out during a public health emergency they should be accompanied by transparent communication between the ECs involved. The MSF ERB experience should trigger a broader discussion on the 'optimal' ethics review in an emergency outbreak and what enduring structural changes are needed to improve the ethics review process.
Figures
References
-
- Ahmad A., Mahmud S. M. (2010). Philanthropic Misconception. Asian Bioethics Review, 2, 154–161.
-
- Baggi F. M., Taybee A., Kurth A., Van Herp M., Di Caro A., Wölfel R., Günther S., Decroo T., Declerck H., Jonckheere S. (2014). Management of Pregnant Women Infected with Ebola Virus in a Treatment Centre in Guinea. Euro Surveillance, 19, pii=20983.. - PubMed
-
- Baize S., Pannetier D., Oestereich L., Rieger T., Koivogui L., Magassouba N. F., Soropogui B., Sow M. S., Keïta S., De Clerck H., Tiffany A., Dominguez G., Loua M., Traoré A., Kolié M., Malano E. R., Heleze E., Bocquin A., Mély S., Raoul H., Caro V., Cadar D., Gabriel M., Pahlmann M., Tappe D., Schmidt-Chanasit J., Impouma B., Diallo A. K., Formenty P., Van Herp M., Günther S. (2014). Emergence of Zaire Ebola Virus Disease in Guinea. New England Journal of Medicine, 371, 1418–1425. - PubMed
-
- Baylis F., Halperin S. A. (2012). Research Involving Pregnant Women: Trials and Tribulations. Clinical Investigation, 2, 139–146.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

