Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: a retrospective expertise-based study from two centers
- PMID: 28567118
- PMCID: PMC5424875
- DOI: 10.1177/1756283X17702096
Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: a retrospective expertise-based study from two centers
Abstract
Background: Percutaneous transhepatic biliary drainage (PTBD) is widely performed as a salvage procedure in patients with unresectable malignant obstruction of the common bile duct (CBD) after failed endoscopic retrograde cholangiopancreatography (ERCP) or in case of surgically altered anatomy. Endoscopic ultrasound-guided hepaticogastrostomy (EU-HGS) is a more recently introduced alternative to relieve malignant obstructive jaundice. The aim of this prospective observational study was to compare the outcome, efficacy and adverse events of EU-HGS and PTBD.
Methods: From April 2012 to August 2015, consecutive patients with malignant CBD obstruction who underwent EU-HGS or PTBD in two tertiary-care referral centers were included. The primary endpoint was the clinical success rate. Secondary endpoints were technical success, overall survival, procedure-related adverse events, incidence of adverse events, and reintervention rate.
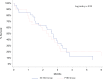
Results: A total of 51 patients (EU-HGS, n = 31; PTBD, n = 20) were included. Median survival was 71 days (range 25-75th percentile; 30-95) for the EU-HGS group and 78 days (range 25-75th percentile; 42-108) for the PTBD group (p = 0.99). Technical success was achieved in all patients in both groups. Clinical success was achieved in 25 (86%) of 31 patients in the EU-HGS group and in 15 (83%) of 20 patients in the PTBD group (p = 0.88). There was no difference in adverse events rates between the two groups (EU-HGS: 16%; PTBD: 10%) (p = 0.69). Four deaths within 1 month (two hemorrhagic and two septic) were considered procedure related (two in the EU-HGS group and two in the PTBD group). Overall reintervention rate was significantly lower after EU-HGS (n = 2) than after PTBD (n = 21) (p = 0.0001). Length of hospital stay was shorter after EU-HGS (8 days versus 15 days; p = 0.002).
Conclusions: EU-HGS can be an effective and safe mini invasive-procedure alternative to PTBD, with similar success and adverse-event rates, but with lower rates of reintervention and length of hospitalization.
Keywords: ERCP; EUS; PTBD; jaundice; pancreatic cancer.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures




References
-
- ASGE guidelines for clinical application. The role of ERCP in diseases of the biliary tract and pancreas. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 1999; 50: 915–920. - PubMed
-
- Fogel EL, Sherman S, Devereaux BM, et al. Therapeutic biliary endoscopy. Endoscopy 2001; 33: 31–38. - PubMed
-
- Ekkelenkamp VE, de Man RA, Ter Borg F, et al. Prospective evaluation of ERCP performance: results of a nationwide quality registry. Endoscopy 2015; 47: 503–507. - PubMed
-
- Baron TH, Petersen BT, Mergener K, et al. Quality indicators for endoscopic retrograde cholangiopancreatography. Gastrointest Endosc 2006; 63 (Suppl. 4): S29–S34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

