Non-bonding 1,5-S···O interactions govern chemo- and enantioselectivity in isothiourea-catalyzed annulations of benzazoles
- PMID: 28567263
- PMCID: PMC5450589
- DOI: 10.1039/c6sc00940a
Non-bonding 1,5-S···O interactions govern chemo- and enantioselectivity in isothiourea-catalyzed annulations of benzazoles
Abstract
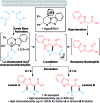
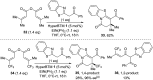
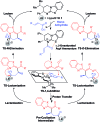
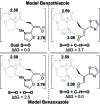
Isothiourea-catalyzed annulations between 2-acyl benzazoles and α,β-unsaturated acyl ammonium intermediates are selectively tuned to form either lactam or lactone heterocycles in good yields (up to 95%) and high ee (up to 99%) using benzothiazole or benzoxazole derivatives, respectively. Computation gives insight into the significant role of two 1,5-S···O interactions in controlling the structural preorganization and chemoselectivity observed within the lactam synthesis with benzothiazoles as nucleophiles. When using benzazoles the absence of a second stabilizing non-bonding 1,5-S···O interaction leads to a dominant C-H···O interaction in determining structural preorganization and lactone formation.
Figures





References
-
- Dua R., Shrivastava S., Sonwane S. K., Srivastava S. K. Adv. Biol. Res. 2011;5:120–144.
- Eicher T. and Hauptmann S., The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications, Wiley-VCH, Weinheim, Germany, 2nd edn, 2003.
-
- Keri R. S., Patil M. R., Patil S. A., Budagumpi S. Eur. J. Med. Chem. 2015;89:207–251. - PubMed
- Noel S., Cadet S., Gras E., Hureau C. Chem. Soc. Rev. 2013;42:7747–7762. - PubMed
- Bansal Y., Silakari O. Bioorg. Med. Chem. 2012;20:6208–6236. - PubMed
- Demmer C. S., Bunch L. Eur. J. Med. Chem. 2014;97:778–785. - PubMed
-
- Rodembusch F. S., Leusin F. P., da Costa Medina L. F., Brandelli A., Stefani V. Photochem. Photobiol. Sci. 2005;4:254–259. - PubMed
-
-
For selected recent examples, see:
- Cai Q., Li Z., Wei J., Fu L., Ha C., Pei D., Ding K. Org. Lett. 2010;12:1500–1503. - PubMed
- De Silva H., Chatterjee S., Henry W. P., Pittman Jr C. U. Synthesis. 2012;44:3453–3464.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

