MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-κB signaling pathway
- PMID: 28569771
- PMCID: PMC5520883
- DOI: 10.1038/cddis.2017.211
MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-κB signaling pathway
Abstract
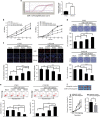
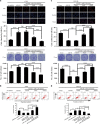
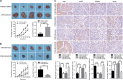
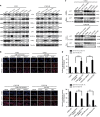
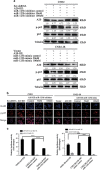
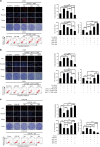
MiR-125b is aberrantly expressed and has a role in the various types of tumors. However, the role and mechanism of miR-125b in nasopharyngeal carcinoma (NPC) are unclear. In this study, we investigated the role and mechanism of miR-125b in NPC. We observed that miR-125b was significantly upregulated in the NPC tissues relative to normal nasopharyngeal mucosa (NNM), and its increment was correlated with poor patient survival, and was an independent predictor for reduced patient survival; miR-125b promoted NPC cell proliferation and inhibited NPC cell apoptosis; in a mouse model, administration of miR-125b antagomir significantly reduced the growth of NPC xenograft tumors. Mechanistically, we confirmed that A20 was a direct target of miR-125b, and found that activation of nuclear factor κB (NF-κB) signaling pathway by A20 mediated miR-125b-promoting NPC cell proliferation and -inhibiting NPC cell apoptosis. With a combination of loss-of-function and gain-of-function approaches, we further showed that A20 inhibited NPC cell proliferation, induced NPC cell apoptosis, and reduced the growth of NPC xenograft tumors. Moreover, A20 was significantly downregulated, whereas p-p65(RelA) was significantly upregulated in the NPC tissues relative to normal nasopharyngeal mucosa, and miR-125b level was negatively associated with A20 level, whereas positively associated with p-p65 level. Our data demonstrate that miR-125b regulates NPC cell proliferation and apoptosis by targeting A20/NF-κB signaling pathway, and miR-125b acts as oncogene, whereas A20 functions as tumor suppressor in NPC, highlighting the therapeutic potential of miR-125b/A20/NF-κB signaling axis in the NPC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol 2014; 11: 145–156. - PubMed
-
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857–866. - PubMed
-
- Yin H, Sun Y, Wang X, Park J, Zhang Y, Li M et al. Progress on the relationship between miR-125 family and tumorigenesis. Exp Cell Res 2015; 339: 252–260. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

