Disease-linked connexin26 S17F promotes volar skin abnormalities and mild wound healing defects in mice
- PMID: 28569788
- PMCID: PMC5520893
- DOI: 10.1038/cddis.2017.234
Disease-linked connexin26 S17F promotes volar skin abnormalities and mild wound healing defects in mice
Erratum in
-
Correction to: Disease-linked connexin26 S17F promotes volar skin abnormalities and mild wound healing defects in mice.Cell Death Dis. 2018 May 24;9(6):630. doi: 10.1038/s41419-018-0639-1. Cell Death Dis. 2018. PMID: 29795380 Free PMC article.
Abstract
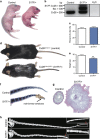
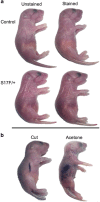
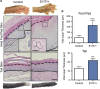
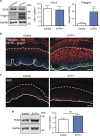
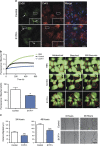
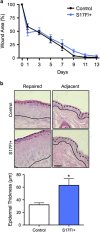
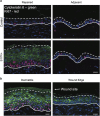
Several mutant mice have been generated to model connexin (Cx)-linked skin diseases; however, the role of connexins in skin maintenance and during wound healing remains to be fully elucidated. Here we generated a novel, viable, and fertile mouse (Cx26CK14-S17F/+) with the keratitis-ichthyosis-deafness mutant (Cx26S17F) driven by the cytokeratin 14 promoter. This mutant mouse mirrors several Cx26-linked human skin pathologies suggesting that the etiology of Cx26-linked skin disease indeed stems from epidermal expression of the Cx26 mutant. Cx26CK14-S17F/+ foot pad epidermis formed severe palmoplantar keratoderma, which expressed elevated levels of Cx26 and filaggrin. Primary keratinocytes isolated from Cx26CK14-S17F/+ neonates exhibited reduced gap junctional intercellular communication and migration. Furthermore, Cx26CK14-S17F/+ mouse skin wound closure was normal but repaired epidermis appeared hyperplastic with elevated expression of cytokeratin 6. Taken together, we suggest that the Cx26S17F mutant disturbs keratinocyte differentiation and epidermal remodeling following wound closure. We further posit that Cx26 contributes to epidermal homeostasis by regulating keratinocyte differentiation, and that mice harboring a disease-linked Cx26 mutant display epidermal abnormalities yet retain most wound healing properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem 2003; 10: 2045–58. - PubMed
-
- Avshalumova L, Fabrikant J, Koriakos A. Overview of skin diseases linked to connexin gene mutations. Int J Dermatol 2014; 53: 192–205. - PubMed
-
- Martin PE, van Steensel M. Connexins and skin disease: insights into the role of beta connexins in skin homeostasis. Cell Tissue Res 2015; 360: 645–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

