Human cancer-associated fibroblasts enhance glutathione levels and antagonize drug-induced prostate cancer cell death
- PMID: 28569790
- PMCID: PMC5520886
- DOI: 10.1038/cddis.2017.225
Human cancer-associated fibroblasts enhance glutathione levels and antagonize drug-induced prostate cancer cell death
Abstract
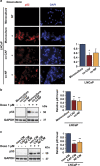
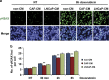
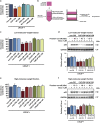
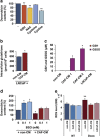
Drug resistance is a major problem in cancer therapy. A growing body of evidence demonstrates that the tumor microenvironment, including cancer-associated fibroblasts (CAFs), can modulate drug sensitivity in tumor cells. We examined the effect of primary human CAFs on p53 induction and cell viability in prostate cancer cells on treatment with chemotherapeutic drugs. Co-culture with prostate CAFs or CAF-conditioned medium attenuated DNA damage and the p53 response to chemotherapeutic drugs and enhanced prostate cancer cell survival. CAF-conditioned medium inhibited the accumulation of doxorubicin, but not taxol, in prostate cancer cells in a manner that was associated with increased cancer cell glutathione levels. A low molecular weight fraction (<3 kDa) of CAF-conditioned medium had the same effect. CAF-conditioned medium also inhibited induction of reactive oxygen species (ROS) in both doxorubicin- and taxol-treated cancer cells. Our findings suggest that CAFs can enhance drug resistance in cancer cells by inhibiting drug accumulation and counteracting drug-induced oxidative stress. This protective mechanism may represent a novel therapeutic target in cancer.
Conflict of interest statement
KGW and VJNB are co-founders and shareholders of Aprea Therapeutics AB, a company that develops novel p53-based cancer therapy. KGW is a member of its Clinical Advisory Board. The remaining authors declare no conflict of interest.
Figures


References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322. - PubMed
-
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004; 6: 17–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

