Thermal annihilation of photo-induced radicals following dynamic nuclear polarization to produce transportable frozen hyperpolarized 13C-substrates
- PMID: 28569840
- PMCID: PMC5461505
- DOI: 10.1038/ncomms15757
Thermal annihilation of photo-induced radicals following dynamic nuclear polarization to produce transportable frozen hyperpolarized 13C-substrates
Abstract
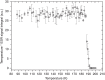
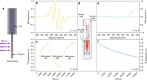
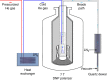
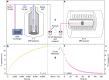
Hyperpolarization via dynamic nuclear polarization (DNP) is pivotal for boosting magnetic resonance imaging (MRI) sensitivity and dissolution DNP can be used to perform in vivo real-time 13C MRI. The type of applications is however limited by the relatively fast decay time of the hyperpolarized spin state together with the constraint of having to polarize the 13C spins in a dedicated apparatus nearby but separated from the MRI magnet. We herein demonstrate that by polarizing 13C with photo-induced radicals, which can be subsequently annihilated using a thermalization process that maintains the sample temperature below its melting point, hyperpolarized 13C-substrates can be extracted from the DNP apparatus in the solid form, while maintaining the enhanced 13C polarization. The melting procedure necessary to transform the frozen solid into an injectable solution containing the hyperpolarized 13C-substrates can therefore be performed ex situ, up to several hours after extraction and storage of the polarized solid.
Conflict of interest statement
A. Comment is currently employed by General Electric Medical Systems, Inc. The remaining authors declare no competing financial interests.
Figures
References
-
- James M. L. & Gambhir S. S. A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev. 92, 897–965 (2012). - PubMed
-
- Comment A. in New Applications of NMR in Drug Discovery and Development 252–272The Royal Society of Chemistry (2013).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

