Characteristics of HIV target CD4 T cells collected using different sampling methods from the genital tract of HIV seronegative women
- PMID: 28570576
- PMCID: PMC5453484
- DOI: 10.1371/journal.pone.0178193
Characteristics of HIV target CD4 T cells collected using different sampling methods from the genital tract of HIV seronegative women
Abstract
Background: Understanding the immune profile of CD4 T cells, the primary targets for HIV, in the female genital tract (FGT) is critical for evaluating and developing effective biomedical HIV prevention strategies in women. However, longitudinal investigation of HIV susceptibility markers expressed by FGT CD4 T cells has been hindered by low cellular yield and risk of sampling-associated trauma. We investigated three minimally invasive FGT sampling methods to characterize and compare CD4 T cell yield and phenotype with the goal of establishing feasible sampling strategies for immune profiling of mucosal CD4 T cells.
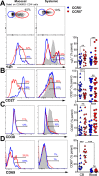
Methods and results: FGT samples were collected bimonthly from 12 healthy HIV negative women of reproductive age in the following order: 1) Cervicovaginal lavage (CVL), 2) two sequential endocervical flocked swabs (FS), and 3) two sequential endocervical cytobrushes (CB1, CB2). Cells were isolated and phentoyped via flow cytometry. CD4 T cell recovery was highest from each individual CB compared to either CVL or FS (p < 0.0001). The majority of CD4 T cells within the FGT, regardless of sampling method, expressed CCR5 relative to peripheral blood (p < 0.01). Within the CB, CCR5+ CD4 T cells expressed significantly higher levels of α4β7, CD69, and low levels of CD27 relative to CCR5- CD4 T cells (all p < 0.001). We also identified CD4 Treg lineage cells expressing CCR5 among CB samples.
Conclusions: Using three different mucosal sampling methods collected longitudinally we demonstrate that CD4 T cells within the FGT express CCR5 and α4β7 and are highly activated, attributes which could act in concert to facilitate HIV acquisition. FS and CB sampling methods can allow for investigation of strategies to reduce HIV target cells in the FGT and could inform the design and interpretation microbicide and vaccine studies in women.
Conflict of interest statement
Figures



References
-
- Mestecky J, Moldoveanu Z, Smith PD, Hel Z, Alexander RC. Mucosal immunology of the genital and gastrointestinal tracts and HIV-1 infection. Journal of reproductive immunology. 2009;83(1–2):196–200. Epub 2009/10/27. PubMed Central PMCID: PMC2802574. doi: 10.1016/j.jri.2009.07.005 - DOI - PMC - PubMed
-
- McKinnon LR, Kaul R. Quality and quantity: mucosal CD4+ T cells and HIV susceptibility. Current opinion in HIV and AIDS. 2012;7(2):195–202. Epub 2012/02/09. doi: 10.1097/COH.0b013e3283504941 - DOI - PubMed
-
- Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26(2):257–70. PubMed Central PMCID: PMCPMC1885958. doi: 10.1016/j.immuni.2007.01.007 - DOI - PMC - PubMed
-
- Yi TJ, Shannon B, Prodger J, McKinnon L, Kaul R. Genital immunology and HIV susceptibility in young women. Am J Reprod Immunol. 2013;69 Suppl 1:74–9. Epub 2012/11/20. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

