Clinical, ultrasound and molecular biomarkers for early prediction of large for gestational age infants in nulliparous women: An international prospective cohort study
- PMID: 28570613
- PMCID: PMC5453528
- DOI: 10.1371/journal.pone.0178484
Clinical, ultrasound and molecular biomarkers for early prediction of large for gestational age infants in nulliparous women: An international prospective cohort study
Abstract
Objective: To develop a prediction model for term infants born large for gestational age (LGA) by customised birthweight centiles.
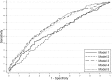
Methods: International prospective cohort of nulliparous women with singleton pregnancy recruited to the Screening for Pregnancy Endpoints (SCOPE) study. LGA was defined as birthweight above the 90th customised centile, including adjustment for parity, ethnicity, maternal height and weight, fetal gender and gestational age. Clinical risk factors, ultrasound parameters and biomarkers at 14-16 or 19-21 weeks were combined into a prediction model for LGA infants at term using stepwise logistic regression in a training dataset. Prediction performance was assessed in a validation dataset using area under the Receiver Operating Characteristics curve (AUC) and detection rate at fixed false positive rates.
Results: The prevalence of LGA at term was 8.8% (n = 491/5628). Clinical and ultrasound factors selected in the prediction model for LGA infants were maternal birthweight, gestational weight gain between 14-16 and 19-21 weeks, and fetal abdominal circumference, head circumference and uterine artery Doppler resistance index at 19-21 weeks (AUC 0.67; 95%CI 0.63-0.71). Sensitivity of this model was 24% and 49% for a fixed false positive rate of 10% and 25%, respectively. The addition of biomarkers resulted in selection of random glucose, LDL-cholesterol, vascular endothelial growth factor receptor-1 (VEGFR1) and neutrophil gelatinase-associated lipocalin (NGAL), but with minimal improvement in model performance (AUC 0.69; 95%CI 0.65-0.73). Sensitivity of the full model was 26% and 50% for a fixed false positive rate of 10% and 25%, respectively.
Conclusion: Prediction of LGA infants at term has limited diagnostic performance before 22 weeks but may have a role in contingency screening in later pregnancy.
Conflict of interest statement
Figures
Similar articles
-
Prediction of Small for Gestational Age Infants in Healthy Nulliparous Women Using Clinical and Ultrasound Risk Factors Combined with Early Pregnancy Biomarkers.PLoS One. 2017 Jan 9;12(1):e0169311. doi: 10.1371/journal.pone.0169311. eCollection 2017. PLoS One. 2017. PMID: 28068394 Free PMC article.
-
Perinatal outcomes in large infants using customised birthweight centiles and conventional measures of high birthweight.Paediatr Perinat Epidemiol. 2012 Nov;26(6):543-52. doi: 10.1111/ppe.12002. Paediatr Perinat Epidemiol. 2012. PMID: 23061690
-
Clinical prediction in early pregnancy of infants small for gestational age by customised birthweight centiles: findings from a healthy nulliparous cohort.PLoS One. 2013 Aug 5;8(8):e70917. doi: 10.1371/journal.pone.0070917. Print 2013. PLoS One. 2013. PMID: 23940665 Free PMC article.
-
Third trimester abdominal circumference, estimated fetal weight and uterine artery doppler for the identification of newborns small and large for gestational age.Eur J Obstet Gynecol Reprod Biol. 2013 Feb;166(2):133-8. doi: 10.1016/j.ejogrb.2012.10.010. Epub 2012 Oct 31. Eur J Obstet Gynecol Reprod Biol. 2013. PMID: 23122032 Clinical Trial.
-
Mid-pregnancy fetal biometry, uterine artery Doppler indices and maternal demographic characteristics: role in prediction of small-for-gestational-age birth.Acta Obstet Gynecol Scand. 2016 Feb;95(2):238-44. doi: 10.1111/aogs.12804. Epub 2015 Nov 8. Acta Obstet Gynecol Scand. 2016. PMID: 26472057
Cited by
-
Preconception and early-pregnancy risk prediction for birth complications: development of prediction models within a population-based prospective cohort.BMC Pregnancy Childbirth. 2022 Feb 28;22(1):165. doi: 10.1186/s12884-022-04497-2. BMC Pregnancy Childbirth. 2022. PMID: 35227240 Free PMC article.
-
Relationships of maternal body mass index and plasma biomarkers with childhood body mass index and adiposity at 6 years: The Children of SCOPE study.Pediatr Obes. 2019 Oct;14(10):e12537. doi: 10.1111/ijpo.12537. Epub 2019 Jun 24. Pediatr Obes. 2019. PMID: 31232532 Free PMC article.
-
Pathologic maternal and neonatal outcomes associated with programmed embryo transfer: potential etiologies and strategies for prevention.J Assist Reprod Genet. 2024 Apr;41(4):843-859. doi: 10.1007/s10815-024-03042-8. Epub 2024 Mar 27. J Assist Reprod Genet. 2024. PMID: 38536596 Free PMC article. Review.
-
White Matter Damage in 4,725 Term-Born Infants Is Determined by Head Circumference at Birth: The Missing Link.Obstet Gynecol Int. 2018 Feb 28;2018:2120835. doi: 10.1155/2018/2120835. eCollection 2018. Obstet Gynecol Int. 2018. PMID: 29681945 Free PMC article.
-
Fetal Genotype and Maternal Glucose Have Independent and Additive Effects on Birth Weight.Diabetes. 2018 May;67(5):1024-1029. doi: 10.2337/db17-1188. Epub 2018 Feb 20. Diabetes. 2018. PMID: 29463506 Free PMC article.
References
-
- Campbell S. Fetal macrosomia: a problem in need of a policy. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014;43(1):3–10. - PubMed
-
- Sanchez-Ramos L, Bernstein S, Kaunitz AM. Expectant management versus labor induction for suspected fetal macrosomia: a systematic review. Obstet Gynecol. 2002;100(5 Pt 1):997–1002. Epub 2002/11/09. - PubMed
-
- Irion O, Boulvain M. Induction of labour for suspected fetal macrosomia. Cochrane Database Syst Rev. 2000;(2):CD000938 Epub 2000/05/05. doi: 10.1002/14651858.CD000938 - DOI - PubMed
-
- Boulvain M, Senat MV, Perrotin F, Winer N, Beucher G, Subtil D, et al. Induction of labour versus expectant management for large-for-date fetuses: a randomised controlled trial. Lancet. 2015;385(9987):2600–5. doi: 10.1016/S0140-6736(14)61904-8 - DOI - PubMed
-
- Goetzinger KR, Tuuli MG, Odibo AO, Roehl KA, Macones GA, Cahill AG. Screening for fetal growth disorders by clinical exam in the era of obesity. J Perinatol. 2013;33(5):352–7. PubMed Central PMCID: PMCPMC3640749. doi: 10.1038/jp.2012.130 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

