Clinical, ultrasound and molecular biomarkers for early prediction of large for gestational age infants in nulliparous women: An international prospective cohort study
- PMID: 28570613
- PMCID: PMC5453528
- DOI: 10.1371/journal.pone.0178484
Clinical, ultrasound and molecular biomarkers for early prediction of large for gestational age infants in nulliparous women: An international prospective cohort study
Abstract
Objective: To develop a prediction model for term infants born large for gestational age (LGA) by customised birthweight centiles.
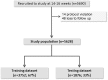
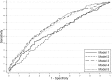
Methods: International prospective cohort of nulliparous women with singleton pregnancy recruited to the Screening for Pregnancy Endpoints (SCOPE) study. LGA was defined as birthweight above the 90th customised centile, including adjustment for parity, ethnicity, maternal height and weight, fetal gender and gestational age. Clinical risk factors, ultrasound parameters and biomarkers at 14-16 or 19-21 weeks were combined into a prediction model for LGA infants at term using stepwise logistic regression in a training dataset. Prediction performance was assessed in a validation dataset using area under the Receiver Operating Characteristics curve (AUC) and detection rate at fixed false positive rates.
Results: The prevalence of LGA at term was 8.8% (n = 491/5628). Clinical and ultrasound factors selected in the prediction model for LGA infants were maternal birthweight, gestational weight gain between 14-16 and 19-21 weeks, and fetal abdominal circumference, head circumference and uterine artery Doppler resistance index at 19-21 weeks (AUC 0.67; 95%CI 0.63-0.71). Sensitivity of this model was 24% and 49% for a fixed false positive rate of 10% and 25%, respectively. The addition of biomarkers resulted in selection of random glucose, LDL-cholesterol, vascular endothelial growth factor receptor-1 (VEGFR1) and neutrophil gelatinase-associated lipocalin (NGAL), but with minimal improvement in model performance (AUC 0.69; 95%CI 0.65-0.73). Sensitivity of the full model was 26% and 50% for a fixed false positive rate of 10% and 25%, respectively.
Conclusion: Prediction of LGA infants at term has limited diagnostic performance before 22 weeks but may have a role in contingency screening in later pregnancy.
Conflict of interest statement
Figures
References
-
- Campbell S. Fetal macrosomia: a problem in need of a policy. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014;43(1):3–10. - PubMed
-
- Sanchez-Ramos L, Bernstein S, Kaunitz AM. Expectant management versus labor induction for suspected fetal macrosomia: a systematic review. Obstet Gynecol. 2002;100(5 Pt 1):997–1002. Epub 2002/11/09. - PubMed
-
- Irion O, Boulvain M. Induction of labour for suspected fetal macrosomia. Cochrane Database Syst Rev. 2000;(2):CD000938 Epub 2000/05/05. doi: 10.1002/14651858.CD000938 - DOI - PubMed
-
- Boulvain M, Senat MV, Perrotin F, Winer N, Beucher G, Subtil D, et al. Induction of labour versus expectant management for large-for-date fetuses: a randomised controlled trial. Lancet. 2015;385(9987):2600–5. doi: 10.1016/S0140-6736(14)61904-8 - DOI - PubMed
-
- Goetzinger KR, Tuuli MG, Odibo AO, Roehl KA, Macones GA, Cahill AG. Screening for fetal growth disorders by clinical exam in the era of obesity. J Perinatol. 2013;33(5):352–7. PubMed Central PMCID: PMCPMC3640749. doi: 10.1038/jp.2012.130 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

