Impact of magnesium:calcium ratio on calcification of the aortic wall
- PMID: 28570619
- PMCID: PMC5453594
- DOI: 10.1371/journal.pone.0178872
Impact of magnesium:calcium ratio on calcification of the aortic wall
Abstract
Objective: An inverse relationship between serum magnesium concentration and vascular calcification has been reported following observational clinical studies. Moreover, several studies have been suggesting a protective effect of magnesium on the vascular calcification. However, the exact mechanism remains elusive, and investigators have speculated among a myriad of potential actions. The effect of magnesium on calcification of the aortic wall is yet to be investigated. In the present study, the effects of magnesium and calcium on the metabolism of extracellular PPi, the main endogenous inhibitor of vascular calcification, were investigated in the rat aorta.
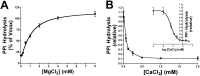
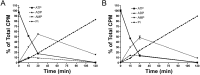
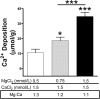
Approach and results: Calcium and magnesium have antagonist effects on PPi hydrolysis in the aortic wall. Km and Ki values for PPi hydrolysis in rat aortic rings were 1.1 mmol/L magnesium and 32 μmol/L calcium, respectively, but ATP hydrolysis was not affected with calcium. Calcium deposition in the rat aortic wall dramatically increased when the magnesium concentration was increased (ratio of Mg:Ca = 1:1; 1.5 mmol/L calcium and 1.5 mmol/L magnesium) respect to low magnesium concentration (ratio Mg:Ca = 1:3, 1.5 mmol/L calcium and 0.75 mmol/L magnesium).
Conclusion: Data from observational clinical studies showing that the serum magnesium concentration is inversely correlated with vascular calcification could be reinterpreted as a compensatory regulatory mechanism that reduces both PPi hydrolysis and vascular calcification. The impact of magnesium in vascular calcification in humans could be studied in association with calcium levels, for example, as the magnesium:calcium ratio.
Conflict of interest statement
Figures


Similar articles
-
Prevention of vascular calcification by polyphosphates and nucleotides- role of ATP.Circ J. 2013;77(8):2145-51. doi: 10.1253/circj.cj-13-0016. Epub 2013 Apr 18. Circ J. 2013. PMID: 23595088
-
Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification.Am J Nephrol. 2012;35(1):31-9. doi: 10.1159/000334742. Epub 2011 Dec 15. Am J Nephrol. 2012. PMID: 22179063
-
Impact of post-dialysis calcium level on ex vivo rat aortic wall calcification.PLoS One. 2017 Aug 23;12(8):e0183730. doi: 10.1371/journal.pone.0183730. eCollection 2017. PLoS One. 2017. PMID: 28832652 Free PMC article.
-
Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium.PLoS One. 2015 Jan 21;10(1):e0115342. doi: 10.1371/journal.pone.0115342. eCollection 2015. PLoS One. 2015. PMID: 25607936 Free PMC article. Review.
-
Relationship between serum magnesium, parathyroid hormone, and vascular calcification in patients on dialysis: a literature review.Perit Dial Int. 2006 May-Jun;26(3):366-73. Perit Dial Int. 2006. PMID: 16722031 Review.
Cited by
-
Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies.Nutrients. 2023 Mar 7;15(6):1304. doi: 10.3390/nu15061304. Nutrients. 2023. PMID: 36986033 Free PMC article.
-
The Relationship between the Concentration of Magnesium and the Presence of Depressive Symptoms and Selected Metabolic Disorders among Men over 50 Years of Age.Life (Basel). 2021 Mar 3;11(3):196. doi: 10.3390/life11030196. Life (Basel). 2021. PMID: 33802529 Free PMC article.
-
Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension.Int J Mol Sci. 2018 Jun 11;19(6):1724. doi: 10.3390/ijms19061724. Int J Mol Sci. 2018. PMID: 29891771 Free PMC article. Review.
-
Effect of vitamin D supplementation on OPG/RANKL signalling activities in endothelial tissue damage in diet-induced diabetic rat model.Pharmacol Rep. 2022 Feb;74(1):124-134. doi: 10.1007/s43440-021-00332-1. Epub 2021 Oct 16. Pharmacol Rep. 2022. PMID: 34657267
-
Magnesium: A Magic Bullet for Cardiovascular Disease in Chronic Kidney Disease?Nutrients. 2019 Feb 22;11(2):455. doi: 10.3390/nu11020455. Nutrients. 2019. PMID: 30813254 Free PMC article. Review.
References
-
- Villa-Bellosta R, Millan A, Sorribas V. Role of calcium-phosphate deposition in vascular smooth muscle cell calcification. Am J Physiol Cell Physiol. 2011. January;300(1):C210–20. doi: 10.1152/ajpcell.00229.2010 - DOI - PubMed
-
- Villa-Bellosta R, Sorribas V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler Thromb Vasc Biol. 2009. May;29(5):761–6. doi: 10.1161/ATVBAHA.108.183384 - DOI - PubMed
-
- Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res. 2011. August 19;109(5):578–92. doi: 10.1161/CIRCRESAHA.111.247965 - DOI - PMC - PubMed
-
- Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acín-Pérez R, Enriquez JA, López-Otín C, et al. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation. 2013. June 18;127(24):2442–51. doi: 10.1161/CIRCULATIONAHA.112.000571 - DOI - PubMed
-
- Villa-Bellosta R, Sorribas V. Calcium phosphate deposition with normal phosphate concentration. -Role of pyrophosphate-. Circ J Off J Jpn Circ Soc. 2011;75(11):2705–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

