Intervention using a novel biodegradable hollow stent containing polylactic acid-polyprolactone-polyethylene glycol complexes against lacrimal duct obstruction disease
- PMID: 28570687
- PMCID: PMC5453559
- DOI: 10.1371/journal.pone.0178679
Intervention using a novel biodegradable hollow stent containing polylactic acid-polyprolactone-polyethylene glycol complexes against lacrimal duct obstruction disease
Abstract
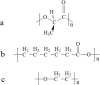
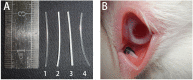
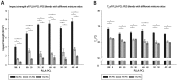
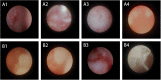
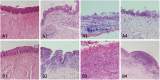
Lacrimal duct obstruction disease (LDOD) is a common ophthalmologic disease. Stent implantation surgery is one of the most effective therapies. In this study, we intended to find out the satisfactory biodegradable stents containing poly-L-lactic acid-polycaprolactone-polyethylene glycol (PLLA- PCL- PEG) complexes for therapeutic application in LDOD. Stents made of PLLA- PCL- PEG complexes in various ratios, were prepared and used in vitro to determine stents with appropriate mechanical properties and shorter range of bio-degradation for study in vivo. Thirty-two rabbits were randomized into eight groups of four eyes each in advance for test in vivo. The selected stents were implanted into the left lacrimal ducts of 16 rabbits and silica gel stents as the control for the other 16 rabbits. At four points in time (1, 4, 10 and 16 weeks after the implantation), weight loss rate (WLR) of the stents was measured and analysed. To access the change of lacrimal duct, fluorescein excretion test, lacrimal duct endoscopy and histopathological testing were conducted. The stent containing PLLA: PCL6: 4+ 15%PEG was selected for study in vivo. Analysis of weight loss rate (WLR), fluorescein excretion test, lacrimal duct endoscopy and histopathological testing indicated that the selected stent was biodegradable and caused minimal stimulation and earlier tissue restoration in the lacrimal epithelium compared with the silica gel stent used as the control. The study results suggest that the PLLA: PCL6: 4+ 15% PEG stent is a satisfactory biodegradable stent as a promising alternative for therapeutic application in LDOD, which showed tissue compatibility, biodegradation and adequate mechanical intensity.
Conflict of interest statement
Figures
References
-
- De Castro DK, Santiago YM, Cunningham M, Gray ST, Metson R, Fay A. A modified lacrimal sac implant for high-risk dacryocystorhinostomy. Ophthalmic plastic and reconstructive surgery. 2013;29(5):367–72. doi: 10.1097/IOP.0b013e31829a72d4 - DOI - PubMed
-
- Samimi DB, Bielory BP, Miller D, Johnson TE. Microbiologic trends and biofilm growth on explanted periorbital biomaterials: a 30-year review. Ophthalmic plastic and reconstructive surgery. 2013;29(5):376–81. doi: 10.1097/IOP.0b013e31829a7313 - DOI - PubMed
-
- Fayet B, Katowitz WR, Racy E, Ruban JM, Katowitz JA. Pushed monocanalicular intubation: an alternative stenting system for the management of congenital nasolacrimal duct obstructions. Journal of AAPOS: the official publication of the American Association for Pediatric Ophthalmology and Strabismus / American Association for Pediatric Ophthalmology and Strabismus. 2012;16(5):468–72. - PubMed
-
- Anderson RL, Edwards JJ. Indications, complications and results with silicone stents. Ophthalmology. 1979;86(8):1474–87. - PubMed
-
- Charalampidou S, Fulcher T. Does the timing of silicone tube removal following external dacryocystorhinostomy affect patients' symptoms? Orbit. 2009;28(2–3):115–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

