Host lipid droplets: An important source of lipids salvaged by the intracellular parasite Toxoplasma gondii
- PMID: 28570716
- PMCID: PMC5469497
- DOI: 10.1371/journal.ppat.1006362
Host lipid droplets: An important source of lipids salvaged by the intracellular parasite Toxoplasma gondii
Abstract
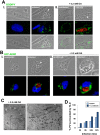
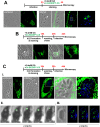
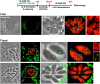
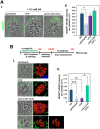
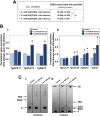
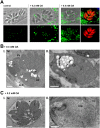
Toxoplasma is an obligate intracellular parasite that replicates in mammalian cells within a parasitophorous vacuole (PV) that does not fuse with any host organelles. One mechanism developed by the parasite for nutrient acquisition is the attraction of host organelles to the PV. Here, we examined the exploitation of host lipid droplets (LD), ubiquitous fat storage organelles, by Toxoplasma. We show that Toxoplasma replication is reduced in host cells that are depleted of LD, or impaired in TAG lipolysis or fatty acid catabolism. In infected cells, the number of host LD and the expression of host LD-associated genes (ADRP, DGAT2), progressively increase until the onset of parasite replication. Throughout infection, the PV are surrounded by host LD. Toxoplasma is capable of accessing lipids stored in host LD and incorporates these lipids into its own membranes and LD. Exogenous addition of oleic acid stimulates LD biogenesis in the host cell and results in the overaccumulation of neutral lipids in very large LD inside the parasite. To access LD-derived lipids, Toxoplasma intercepts and internalizes within the PV host LD, some of which remaining associated with Rab7, which become wrapped by an intravacuolar network of membranes (IVN). Mutant parasites impaired in IVN formation display diminished capacity of lipid uptake from host LD. Moreover, parasites lacking an IVN-localized phospholipase A2 are less proficient in salvaging lipids from host LD in the PV, suggesting a major contribution of the IVN for host LD processing in the PV and, thus lipid content release. Interestingly, gavage of parasites with lipids unveils, for the first time, the presence in Toxoplasma of endocytic-like structures containing lipidic material originating from the PV lumen. This study highlights the reliance of Toxoplasma on host LD for its intracellular development and the parasite's capability in scavenging neutral lipids from host LD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Stone SS, Levin MC, Zhou P, Han J, Walther TC, Farese RV Jr (2009) The endoplasmic reticulum enzyme, DGAT2, is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem 284: 5352–5361. 10.1074/jbc.M805768200 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

