Quinary interactions with an unfolded state ensemble
- PMID: 28571108
- PMCID: PMC5563149
- DOI: 10.1002/pro.3206
Quinary interactions with an unfolded state ensemble
Abstract
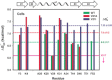
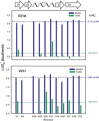
Anfinsen's thermodynamic hypothesis states that the native three-dimensional fold of a protein represents the structure with the lowest Gibbs free energy. Changes in the free energy of denaturation can arise from changes to the folded state, the unfolded state, or both. It has been recently recognized that quinary interactions, transient contacts that take place only in cells, can modulate protein stability through interactions involving the folded state. Here we show that the cellular environment can also remodel the unfolded state ensemble.
Keywords: NMR; amide proton exchange; folding; quinary structure; stability; thermodynamics; unfolded ensemble.
© 2017 The Protein Society.
Figures


Similar articles
-
Unfolded protein ensembles, folding trajectories, and refolding rate prediction.J Chem Phys. 2013 Sep 28;139(12):121925. doi: 10.1063/1.4817215. J Chem Phys. 2013. PMID: 24089737
-
Effect of salt on the urea-unfolded form of barstar probed by m value measurements.Biochemistry. 2004 Sep 14;43(36):11393-402. doi: 10.1021/bi049320b. Biochemistry. 2004. PMID: 15350126
-
Monitoring protein unfolding transitions by NMR-spectroscopy.J Biomol NMR. 2022 Apr;76(1-2):3-15. doi: 10.1007/s10858-021-00389-3. Epub 2022 Jan 4. J Biomol NMR. 2022. PMID: 34984658 Free PMC article.
-
Statistical Thermodynamics of the Protein Ensemble: Mediating Function and Evolution.Annu Rev Biophys. 2025 May;54(1):227-247. doi: 10.1146/annurev-biophys-061824-104900. Epub 2025 Feb 10. Annu Rev Biophys. 2025. PMID: 39929551 Review.
-
Where the complex things are: single molecule and ensemble spectroscopic investigations of protein folding dynamics.Curr Opin Struct Biol. 2016 Feb;36:1-9. doi: 10.1016/j.sbi.2015.11.006. Epub 2015 Dec 11. Curr Opin Struct Biol. 2016. PMID: 26687767 Review.
Cited by
-
Both Ligands and Macromolecular Crowders Preferentially Bind to Closed Conformations of Maltose Binding Protein.Biochemistry. 2019 Apr 30;58(17):2208-2217. doi: 10.1021/acs.biochem.9b00154. Epub 2019 Apr 12. Biochemistry. 2019. PMID: 30950267 Free PMC article.
-
Osmolytes and crowders regulate aggregation of the cancer-related L106R mutant of the Axin protein.Biophys J. 2021 Aug 17;120(16):3455-3469. doi: 10.1016/j.bpj.2021.05.024. Epub 2021 Jun 2. Biophys J. 2021. PMID: 34087214 Free PMC article.
-
Direct activation of HSF1 by macromolecular crowding and misfolded proteins.PLoS One. 2024 Nov 4;19(11):e0312524. doi: 10.1371/journal.pone.0312524. eCollection 2024. PLoS One. 2024. PMID: 39495731 Free PMC article.
-
Surface Charge Modulates Protein-Protein Interactions in Physiologically Relevant Environments.Biochemistry. 2018 Mar 20;57(11):1681-1684. doi: 10.1021/acs.biochem.8b00061. Epub 2018 Mar 6. Biochemistry. 2018. PMID: 29473738 Free PMC article.
-
Surface Attachment Enhances the Thermodynamic Stability of Protein L.Angew Chem Int Ed Engl. 2019 Feb 4;58(6):1714-1718. doi: 10.1002/anie.201812231. Epub 2019 Jan 15. Angew Chem Int Ed Engl. 2019. PMID: 30549169 Free PMC article.
References
-
- Fisher E (1894) Einfluss der configuration auf die wirkung den enzyme. Ber Dtsch Chem Ges 27:2985–2993.
-
- Edsall JT (1995) Hsien Wu and the first theory of protein denaturation (1931). Adv Protein Chem 46:1–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

