Comparison of in vitro toxicological effects of biomass smoke from different sources of animal dung
- PMID: 28572013
- PMCID: PMC5736384
- DOI: 10.1016/j.tiv.2017.05.021
Comparison of in vitro toxicological effects of biomass smoke from different sources of animal dung
Abstract
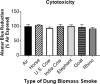
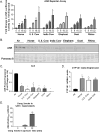
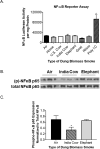
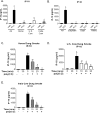
Worldwide, over 4 million premature deaths each year are attributed to the burning of biomass fuels for cooking and heating. Epidemiological studies associate household air pollution with lung diseases, including chronic obstructive pulmonary disease, lung cancer, and respiratory infections. Animal dung, a biomass fuel used by economically vulnerable populations, generates more toxic compounds per mass burned than other biomass fuels. The type of animal dung used varies widely depending on local agro-geography. There are currently neither standardized experimental systems for dung biomass smoke research nor studies assessing the health impacts of different types of dung smoke. Here, we used a novel reproducible exposure system to assess outcomes related to inflammation and respiratory infections in human airway cells exposed to six different types of dung biomass smoke. We report that dung biomass smoke, regardless of species, is pro-inflammatory and activates the aryl hydrocarbon receptor and JNK transcription factors; however, dung smoke also suppresses interferon responses after a challenge with a viral mimetic. These effects are consistent with epidemiological data, and suggest a mechanism by which the combustion of animal dung can directly cause lung diseases, promote increased susceptibility to infection, and contribute to the global health problem of household air pollution.
Keywords: Biomass smoke; Household air pollution; Respiratory toxicology.
Copyright © 2017. Published by Elsevier Ltd.
Figures





Similar articles
-
Dung biomass smoke activates inflammatory signaling pathways in human small airway epithelial cells.Am J Physiol Lung Cell Mol Physiol. 2016 Dec 1;311(6):L1222-L1233. doi: 10.1152/ajplung.00183.2016. Epub 2016 Nov 11. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27836898 Free PMC article.
-
Dung biomass smoke exposure impairs resolution of inflammatory responses to influenza infection.Toxicol Appl Pharmacol. 2022 Sep 1;450:116160. doi: 10.1016/j.taap.2022.116160. Epub 2022 Jul 9. Toxicol Appl Pharmacol. 2022. PMID: 35817128 Free PMC article.
-
Biomass Smoke Exposure Enhances Rhinovirus-Induced Inflammation in Primary Lung Fibroblasts.Int J Mol Sci. 2016 Aug 25;17(9):1403. doi: 10.3390/ijms17091403. Int J Mol Sci. 2016. PMID: 27571064 Free PMC article.
-
Exposure to biomass smoke as a cause for airway disease in women and children.Curr Opin Allergy Clin Immunol. 2012 Feb;12(1):82-90. doi: 10.1097/ACI.0b013e32834ecb65. Curr Opin Allergy Clin Immunol. 2012. PMID: 22157154 Review.
-
Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions.Environ Health Perspect. 2007 Jun;115(6):848-55. doi: 10.1289/ehp.9479. Epub 2007 Feb 27. Environ Health Perspect. 2007. PMID: 17589590 Free PMC article. Review.
Cited by
-
Sex-Dependent Influence of Developmental Toxicant Exposure on Group B Streptococcus-Mediated Preterm Birth in a Murine Model.Reprod Sci. 2018 May;25(5):662-673. doi: 10.1177/1933719117741378. Epub 2017 Nov 19. Reprod Sci. 2018. PMID: 29153057 Free PMC article.
-
COVID-19 Susceptibility in chronic obstructive pulmonary disease.Eur J Clin Invest. 2020 Oct;50(10):e13382. doi: 10.1111/eci.13382. Epub 2020 Sep 2. Eur J Clin Invest. 2020. PMID: 32780415 Free PMC article. Review.
-
Evidence of Biomass Smoke Exposure as a Causative Factor for the Development of COPD.Toxics. 2017 Dec 1;5(4):36. doi: 10.3390/toxics5040036. Toxics. 2017. PMID: 29194400 Free PMC article. Review.
-
Toxicity of particles derived from combustion of Ethiopian traditional biomass fuels in human bronchial and macrophage-like cells.Arch Toxicol. 2024 May;98(5):1515-1532. doi: 10.1007/s00204-024-03692-8. Epub 2024 Mar 1. Arch Toxicol. 2024. PMID: 38427118 Free PMC article.
-
Environmental pollutants and their effects on human health.Heliyon. 2023 Aug 25;9(9):e19496. doi: 10.1016/j.heliyon.2023.e19496. eCollection 2023 Sep. Heliyon. 2023. PMID: 37662771 Free PMC article. Review.
References
-
- Yadama GN. Fires, fuel, and the fate of 3 billion: The state of the energy impoverished. Oxford: Oxford University Press; 2013.
-
- World Health Organization. Burden of disease from household air pollution for 2012. 2014
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

