GDF15 is a heart-derived hormone that regulates body growth
- PMID: 28572090
- PMCID: PMC5538424
- DOI: 10.15252/emmm.201707604
GDF15 is a heart-derived hormone that regulates body growth
Abstract
The endocrine system is crucial for maintaining whole-body homeostasis. Little is known regarding endocrine hormones secreted by the heart other than atrial/brain natriuretic peptides discovered over 30 years ago. Here, we identify growth differentiation factor 15 (GDF15) as a heart-derived hormone that regulates body growth. We show that pediatric heart disease induces GDF15 synthesis and secretion by cardiomyocytes. Circulating GDF15 in turn acts on the liver to inhibit growth hormone (GH) signaling and body growth. We demonstrate that blocking cardiomyocyte production of GDF15 normalizes circulating GDF15 level and restores liver GH signaling, establishing GDF15 as a bona fide heart-derived hormone that regulates pediatric body growth. Importantly, plasma GDF15 is further increased in children with concomitant heart disease and failure to thrive (FTT). Together these studies reveal a new endocrine mechanism by which the heart coordinates cardiac function and body growth. Our results also provide a potential mechanism for the well-established clinical observation that children with heart diseases often develop FTT.
Keywords: GDF15; body growth; failure to thrive; heart disease; heart‐derived hormone.
© 2017 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

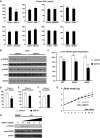
- A
Daily body weight of αKOγKO and littermate control mice. n = 55–145 mice per group with both genders included.
- B
Representative picture of 10‐day‐old αKOγKO and littermate control αHetγWT mice.
- C, D
Plasma GH and IGF1 concentrations in 10‐ (C, n = 7–10 mice per group) and 16‐day‐old mice (D, n = 9–11 mice per group) measured by ELISA.
- E
Phosphorylated (Tyr694) and total STAT5 in 10‐day‐old mouse livers determined by Western blot (n = 3–4 mice per group). β‐Actin serves as a loading control.
- F
Relative levels of phosphorylated and total STAT5 and JAK2 in 13‐day‐old mouse livers (normalized to total protein content of individual mouse liver) were quantified by ELISA (n = 13 mice per group).
- G
Expression of STAT5 target genes Igf1, Igfbp3, and Igfals in 10‐day‐old mouse livers measured by qPCR (n = 7–8 mice per group).
- H
Liver (normalized to total protein content, n = 15–16 mice per group) and plasma (n = 9–10 mice per group) IGFBP3 concentrations in 13‐day‐old mice measured by ELISA.

- A, B
Phosphorylated and total STAT5 in WT mouse primary hepatocytes treated with DMEM (control), 50% plasma in DMEM (A), or 50% plasma fractions in DMEM (B) for 1 h were determined by Western blot. β‐Actin is used as loading control in all Western blots.
- A
Plasma IGF1 concentrations (ng/ml) in 7‐day‐old weight‐ and gender‐matched littermate WT mice injected with control or different proteins were measured by ELISA (n = 3–5 mice per group, daily i.p. injection from 5 days of age).
- B–G
Liver phosphorylated and total STAT5 and JAK2 as well as β‐actin (loading control) determined by Western blot (B); liver expression of Igf1, Igfbp3, and Igfals quantified by qPCR (C); plasma IGF1 (D), IGFBP3 (E), and GH concentrations (F) measured by ELISA; and daily body weight (G) in weight‐ and gender‐matched littermate WT mice injected with control or GDF15 (n = 5 per group, daily i.p. injection from 3 days of age).
- H
Overnight‐fasted (in DMEM) WT mouse primary hepatocytes were first treated with different concentrations of GDF15 for 30 min and then with 20 ng/ml GH for 15 min. Cellular levels of phosphorylated STAT5, total STAT5, and β‐actin (loading control) were determined by Western blot.
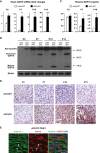
Expression of Gdf15 in 3‐ (n = 5–7 mice per group), 7‐ (n = 6 mice per group), 10‐ (n = 8–10 mice per group), and 13‐day‐old (n = 12–13 mice per group) littermate mouse hearts quantified by qPCR.
GDF15 protein level in 3‐, 7‐, 10‐, and 13‐day‐old littermate mouse hearts determined by Western blot. β‐Actin serves as a loading control.
Plasma GDF15 concentrations in 3‐ (n = 5–7 mice per group), 7‐ (n = 6 mice per group), and 10‐day‐old littermate mice (n = 8–10 mice per group) measured by ELISA.
Representative pictures of 3‐, 7‐, 10‐, and 13‐day‐old littermate αHetγWT and αKOγKO mouse heart sections stained with GDF15 antibody (brown) and counterstained with hematoxylin (purple). Scale bar: 100 μm.
Representative pictures of 16‐day‐old αKOγKO mouse hearts stained with GDF15 (red) and cardiac troponin I (green) antibodies. Arrows point to the nucleus of cardiomyocytes, and asterisks mark the nucleus of non‐cardiomyocytes. Scale bar: 20 μm.

- A
Design of AAV9‐mGDF15 shRNA construct to specifically knockdown GDF15 in Cre+ cardiomyocytes.
- B–G
Cardiac Gdf15 expression quantified by qPCR (B), plasma GDF15 concentrations measured by ELISA (C), cardiac Bnp expression quantified by qPCR (D), liver phosphorylated STAT5 level measured by ELISA (E), and plasma IGF1 (F) and GH concentrations (G) measured by ELISA in 9‐ to 10‐day‐old littermate control and αKOγKO mice (n = 8–12 mice per group) that received pericardial injection of AAV9‐control or Gdf15 shRNA at 2 days of age. *P < 0.05, **P < 0.01, and ***P < 0.001 by t‐test. Values are mean + s.e.m.

Plasma GDF15 concentrations in 2‐ to 3‐year‐old children diagnosed with heart disease with either normal body weight (n = 35) or FTT (n = 45) and in age‐ and gender‐matched healthy controls (n = 45) were measured by ELISA. *P < 0.05 and ***P < 0.001 by t‐test. Values are mean ± s.e.m.
Cartoon illustrating how GDF15 and ANP/BNP relieve cardiac burden and coordinate cardiac function with the rest of the body.
References
-
- Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE (2001) Cyclooxygenase inhibitors regulate the expression of a TGF‐beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol 59: 901–908 - PubMed
-
- Baggen VJ, van den Bosch AE, Eindhoven JA, Schut AW, Cuypers JA, Witsenburg M, de Waart M, van Schaik RH, Zijlstra F, Boersma E et al (2017) Prognostic value of N‐terminal Pro‐B‐type natriuretic peptide, Troponin‐T, and growth‐differentiation factor 15 in adult congenital heart disease. Circulation 135: 264–279 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

