Maternal Exercise Improves Glucose Tolerance in Female Offspring
- PMID: 28572303
- PMCID: PMC5521858
- DOI: 10.2337/db17-0098
Maternal Exercise Improves Glucose Tolerance in Female Offspring
Abstract
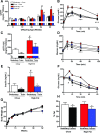
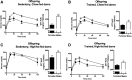
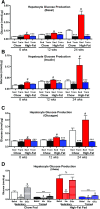
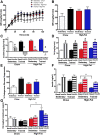
Poor maternal diet can lead to metabolic disease in offspring, whereas maternal exercise may have beneficial effects on offspring health. In this study, we determined ifmaternal exercise could reverse the detrimental effects of maternal high-fat feeding on offspring metabolism of female mice. C57BL/6 female mice were fed a chow (21%) or high-fat (60%) diet and further divided by housing in static cages or cages with running wheels for 2 weeks prior to breeding and throughout gestation. Females were bred with chow-fed sedentary C57BL/6 males. High fat-fed sedentary dams produced female offspring with impaired glucose tolerance compared with offspring of chow-fed dams throughout their first year of life, an effect not present in the offspring from high fat-fed dams that had trained. Offspring from high fat-fed trained dams had normalized glucose tolerance, decreased fasting insulin, and decreased adiposity. Liver metabolic function, measured by hepatic glucose production in isolated hepatocytes, hyperinsulinemic-euglycemic clamps, liver triglyceride content, and liver enzyme expression, was enhanced in offspring from trained dams. In conclusion, maternal exercise negates the detrimental effects of a maternal high-fat diet on glucose tolerance and hepatocyte glucose metabolism in female offspring. The ability of maternal exercise to improve the metabolic health of female offspring is important, as this intervention could combat the transmission of obesity and diabetes to subsequent generations.
© 2017 by the American Diabetes Association.
Figures
References
-
- National Task Force on the Prevention and Treatment of Obesity Overweight, obesity, and health risk. Arch Intern Med 2000;160:898–904 - PubMed
-
- Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, et al. . Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes 2005;54:702–711 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

