Complement-induced activation of MAPKs and Akt during sepsis: role in cardiac dysfunction
- PMID: 28572445
- PMCID: PMC5572692
- DOI: 10.1096/fj.201700140R
Complement-induced activation of MAPKs and Akt during sepsis: role in cardiac dysfunction
Abstract
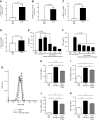
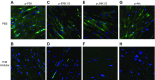
Polymicrobial sepsis in mice causes myocardial dysfunction after generation of the complement anaphylatoxin, complement component 5a (C5a). C5a interacts with its receptors on cardiomyocytes (CMs), resulting in redox imbalance and cardiac dysfunction that can be functionally measured and quantitated using Doppler echocardiography. In this report we have evaluated activation of MAPKs and Akt in CMs exposed to C5a in vitro and after cecal ligation and puncture (CLP) in vivo In both cases, C5a in vitro caused activation (phosphorylation) of MAPKs and Akt in CMs, which required availability of both C5a receptors. Using immunofluorescence technology, activation of MAPKs and Akt occurred in left ventricular (LV) CMs, requiring both C5a receptors, C5aR1 and -2. Use of a water-soluble p38 inhibitor curtailed activation in vivo of MAPKs and Akt in LV CMs as well as the appearance of cytokines and histones in plasma from CLP mice. When mouse macrophages were exposed in vitro to LPS, activation of MAPKs and Akt also occurred. The copresence of the p38 inhibitor blocked these activation responses. Finally, the presence of the p38 inhibitor in CLP mice reduced the development of cardiac dysfunction. These data suggest that polymicrobial sepsis causes cardiac dysfunction that appears to be linked to activation of MAPKs and Akt in heart.-Fattahi, F., Kalbitz, M., Malan, E. A., Abe, E., Jajou, L., Huber-Lang, M. S., Bosmann, M., Russell, M. W., Zetoune, F. S., Ward, P. A. Complement-induced activation of MAPKs and Akt during sepsis: role in cardiac dysfunction.
Keywords: C5a receptor; CLP; cardiomyocyte.
© FASEB.
Figures




References
-
- Fernandes C. J. Jr., Akamine N., Knobel E. (1999) Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Med. 25, 1165–1168 - PubMed
-
- Blanco J., Muriel-Bombín A., Sagredo V., Taboada F., Gandía F., Tamayo L., Collado J., García-Labattut A., Carriedo D., Valledor M., De Frutos M., López M. J., Caballero A., Guerra J., Alvarez B., Mayo A., Villar J.; Grupo de Estudios y Análisis en Cuidados Intensivos (2008) Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit. Care 12, R158 - PMC - PubMed
-
- Nakae H., Endo S., Inada K., Takakuwa T., Kasai T., Yoshida M. (1994) Serum complement levels and severity of sepsis. Res. Commun. Chem. Pathol. Pharmacol. 84, 189–195 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

