Visual Detection of Denatured Glutathione Peptides: A Facile Method to Visibly Detect Heat Stressed Biomolecules
- PMID: 28572597
- PMCID: PMC5453926
- DOI: 10.1038/s41598-017-02899-3
Visual Detection of Denatured Glutathione Peptides: A Facile Method to Visibly Detect Heat Stressed Biomolecules
Abstract
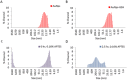
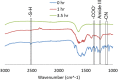
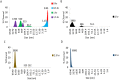
Every year pharmaceutical companies use significant resources to mitigate aggregation of pharmaceutical drug products. Specifically, peptides and proteins that have been denatured or degraded can lead to adverse patient reactions such as undesired immune responses. Current methods to detect aggregation of biological molecules are limited to costly and time consuming processes such as high pressure liquid chromatography, ultrahigh pressure liquid chromatography and SDS-PAGE gels. Aggregation of pharmaceutical drug products can occur during manufacturing, processing, packaging, shipment and storage. Therefore, a facile in solution detection method was evaluated to visually detect denatured glutathione peptides, utilizing gold nanoparticle aggregation via 3-Aminopropyltreithoxysilane. Glutathione was denatured using a 70 °C water bath to create an accelerated heat stressed environment. The peptide, gold nanoparticle and aminosilane solution was then characterized via, UV-Vis spectroscopy, FTIR spectroscopy, dynamic light scattering and scanning electron microscopy. Captured images and resulting absorbance spectra of the gold nanoparticle, glutathione, and aminosilane complex demonstrated visual color changes detectable with the human eye as a function of the denaturation time. This work serves as an extended proof of concept for fast in solution detection methods for glutathione peptides that have experienced heat stress.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Optical Detection of Denatured Ferritin Protein via Plasmonic Gold Nanoparticles Exposure through Aminosilane Solution.Nanomaterials (Basel). 2019 Oct 4;9(10):1417. doi: 10.3390/nano9101417. Nanomaterials (Basel). 2019. PMID: 31590297 Free PMC article.
-
Biomolecule induced nanoparticle aggregation: effect of particle size on interparticle coupling.J Colloid Interface Sci. 2007 Sep 15;313(2):724-34. doi: 10.1016/j.jcis.2007.04.069. Epub 2007 May 3. J Colloid Interface Sci. 2007. PMID: 17540397
-
Interaction of a small heat shock protein of the fission yeast, Schizosaccharomyces pombe, with a denatured protein at elevated temperature.J Biol Chem. 2005 Sep 23;280(38):32586-93. doi: 10.1074/jbc.M504121200. Epub 2005 Jul 29. J Biol Chem. 2005. PMID: 16055437
-
Cysteine-capped gold nanoparticles suppress aggregation of proteins exposed to heat stress.IUBMB Life. 2013 May;65(5):454-61. doi: 10.1002/iub.1146. Epub 2013 Feb 22. IUBMB Life. 2013. PMID: 23436466
-
Impact of nanoparticles on amyloid peptide and protein aggregation: a review with a focus on gold nanoparticles.Nanoscale. 2018 Dec 7;10(45):20894-20913. doi: 10.1039/c8nr04506b. Epub 2018 Sep 18. Nanoscale. 2018. PMID: 30225490 Review.
Cited by
-
Cyanophycin modifications for applications in tissue scaffolding.Appl Microbiol Biotechnol. 2024 Mar 15;108(1):264. doi: 10.1007/s00253-024-13088-4. Appl Microbiol Biotechnol. 2024. PMID: 38489042 Free PMC article.
-
Synergistic In Vitro Anticancer Toxicity of Pulsed Electric Fields and Glutathione.Int J Mol Sci. 2022 Nov 25;23(23):14772. doi: 10.3390/ijms232314772. Int J Mol Sci. 2022. PMID: 36499100 Free PMC article.
-
Colorimetric and Label-Free Optical Detection of Pb2+ Ions via Colloidal Gold Nanoparticles.Biosensors (Basel). 2023 Aug 15;13(8):819. doi: 10.3390/bios13080819. Biosensors (Basel). 2023. PMID: 37622904 Free PMC article.
-
Utilizing polymer-conjugate albumin-based ultrafine gas bubbles in combination with ultra-high frequency radiations in drug transportation and delivery.RSC Adv. 2021 Oct 25;11(55):34440-34448. doi: 10.1039/d1ra04983f. eCollection 2021 Oct 25. RSC Adv. 2021. PMID: 35494740 Free PMC article.
-
Decoding the Virtual 2D Map of the Chloroplast Proteomes.Biol Proced Online. 2022 Dec 13;24(1):23. doi: 10.1186/s12575-022-00186-8. Biol Proced Online. 2022. PMID: 36513972 Free PMC article.
References
-
- O’Connor, C. M. & Adams, J. U. Essentials of Cell Biology. Cambridge, M.A: Ed. (2010).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

