The effect of microbial challenge on the intestinal proteome of broiler chickens
- PMID: 28572745
- PMCID: PMC5450085
- DOI: 10.1186/s12953-017-0118-0
The effect of microbial challenge on the intestinal proteome of broiler chickens
Abstract
Background: In poultry production intestinal health and function is paramount to achieving efficient feed utilisation and growth. Uncovering the localised molecular mechanisms that occur during the early and important periods of growth that allow birds to grow optimally is important for this species. The exposure of young chicks to used litter from older flocks, containing mixed microbial populations, is a widely utilised model in poultry research. It rarely causes mortality but effects an immunogenic stimulation sufficient enough to cause reduced and uneven growth that is reflective of a challenging growing environment.
Methods: A mixed microbial challenge was delivered as used litter containing Campylobacter jejuni and coccidial oocysts to 120 male Ross 308 broiler chicks, randomly divided into two groups: control and challenged. On day 12, 15, 18 and 22 (pre- and 3, 6 and 10 days post-addition of the used litter) the proximal jejunum was recovered from 6 replicates per group and differentially abundant proteins identified between groups and over time using 2D DiGE.
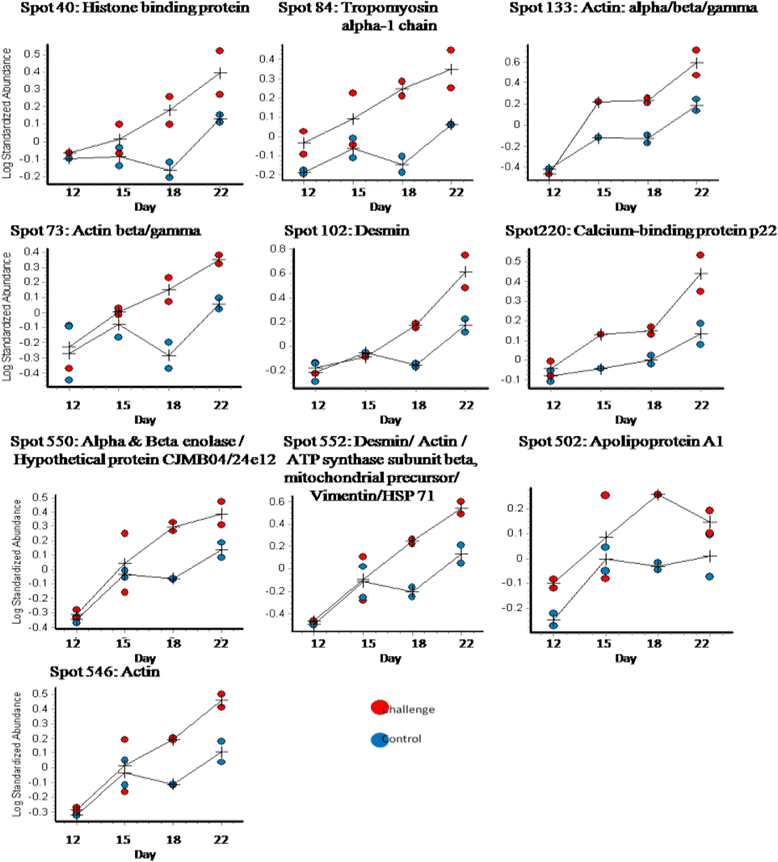
Results: The abundance of cytoskeletal proteins of the chicken small intestinal proteome, particularly actin and actin associated proteins, increased over time in both challenged and control birds. Villin-1, an actin associated anti-apoptotic protein, was reduced in abundance in the challenged birds indicating that many of the changes in cytoskeletal protein abundance in the challenged birds were as a result of an increased rate of apoptosis. A number of heat shock proteins decreased in abundance over time in the intestine and this was more pronounced in the challenged birds.
Conclusions: The small intestinal proteome sampled from 12 to 22 days of age showed considerable developmental change, comparable to other species indicating that many of the changes in protein abundance in the small intestine are conserved among vertebrates. Identifying and distinguishing the changes in proteins abundance and molecular pathways that occur as a result of normal growth from those that occur as a result of a challenging microbial environment is important in this major food producing animal.
Keywords: Apoptosis; Chicken; Intestine; Proteome; Villin.
Figures






References
-
- Davison F, Kaspers B, Schat K. The Avian Mucosal Immune System. In: Davison F, Kaspers B, Schat K, editors. Avian Immunology. Philadelphia: Elsevier Ltd; 2008.
-
- Korver D. Overview of the immune dynamics of the digestive system. J Appl Poult Res. 2006;15:123–135. doi: 10.1093/japr/15.1.123. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

