Host-Parasite Interactions in Human Malaria: Clinical Implications of Basic Research
- PMID: 28572796
- PMCID: PMC5435807
- DOI: 10.3389/fmicb.2017.00889
Host-Parasite Interactions in Human Malaria: Clinical Implications of Basic Research
Abstract
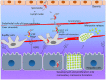
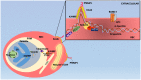
The malaria parasite, Plasmodium, is one of the oldest parasites documented to infect humans and has proven particularly hard to eradicate. One of the major hurdles in designing an effective subunit vaccine against the malaria parasite is the insufficient understanding of host-parasite interactions within the human host during infections. The success of the parasite lies in its ability to evade the human immune system and recruit host responses as physiological cues to regulate its life cycle, leading to rapid acclimatization of the parasite to its immediate host environment. Hence understanding the environmental niche of the parasite is crucial in developing strategies to combat this deadly infectious disease. It has been increasingly recognized that interactions between parasite proteins and host factors are essential to establishing infection and virulence at every stage of the parasite life cycle. This review reassesses all of these interactions and discusses their clinical importance in designing therapeutic approaches such as design of novel vaccines. The interactions have been followed from the initial stages of introduction of the parasite under the human dermis until asexual and sexual blood stages which are essential for transmission of malaria. We further classify the interactions as "direct" or "indirect" depending upon their demonstrated ability to mediate direct physical interactions of the parasite with host factors or their indirect manipulation of the host immune system since both forms of interactions are known to have a crucial role during infections. We also discuss the many ways in which this understanding has been taken to the field and the success of these strategies in controlling human malaria.
Keywords: Plasmodium; cytokines; direct interaction; host–parasite interaction; indirect interaction; invasion; malaria; protein.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

