Prolaris Cell Cycle Progression Test for Localized Prostate Cancer: A Health Technology Assessment
- PMID: 28572867
- PMCID: PMC5451271
Prolaris Cell Cycle Progression Test for Localized Prostate Cancer: A Health Technology Assessment
Abstract
Background: Prostate cancer is very common and many localized tumours are non-aggressive. Determining which cancers are aggressive is important for choosing the most appropriate treatment (e.g., surgery, radiation, active surveillance). Current clinical risk stratification is reliable in forecasting the prognosis of groups of men with similar clinical and pathologic characteristics, but there is residual uncertainty at the individual level. The Prolaris cell cycle progression (CCP) test, a genomic test that estimates how fast tumour cells are proliferating, could potentially be used to improve the accuracy of individual risk assessment. This health technology assessment sought to determine the clinical utility, economic impact, and patients' perceptions of the value of the CCP test in low- and intermediate-risk localized prostate cancer.
Methods: We conducted a systematic review of the clinical and economic evidence of the CCP test in low-and intermediate-risk, localized prostate cancer. Medical and health economic databases were searched from 2010 to June or July 2016. The critical appraisal of the clinical evidence included risk of bias and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We also analyzed the potential budget impact of adding the CCP test into current practice, from the perspective the Ontario Ministry of Health and Long-Term Care. Finally, we conducted qualitative interviews with men with prostate cancer, on the factors that influenced their treatment decision-making.
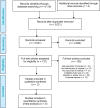
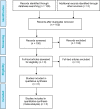
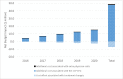
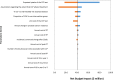
Results: For the review of clinical effectiveness, we screened 3,021 citations, and two before-after studies met our inclusion criteria. In one study, the results of the CCP test appeared to change the treatment plan (from initial to final plan) in 64.9% of cases overall (GRADE rating of the quality of evidence: Very low). In the other study, the CCP test changed the treatment received in nearly half of cases overall, compared with the initial plan (GRADE: Very low). No evidence was available on clinical outcomes of patients whose treatment was informed by CCP results. For the review of cost-effectiveness, 100 citations were identified and screened. No studies met the inclusion criteria. In our economic evaluation, we estimated that publicly funding the CCP test would result in a total net budget impact of $41.3 million in the first 5 years, mostly due to the cost of the CCP test. In our model, the relatively small cost savings ($7.3 million) due to treatment change (increased use of active surveillance and decreased use of interventional treatment) was not large enough to offset the high cost of the test. Patients viewed the test as potentially helpful but, due to the complexity of treatment decision-making, were unsure the test would ultimately change their treatment choices.
Conclusions: We found no evidence to demonstrate the impact of the Prolaris CCP test on patient-important clinical outcomes. The limited evidence available shows that the test appears to provide information that, when considered in addition to clinical risk stratification, may change the treatment plan or actual treatment for some low- and intermediate-risk prostate cancer patients. As a result, there is insufficient data to inform the cost-effectiveness of the CCP test. Publicly funding the CCP test would result in a large incremental cost to the provincial budget.
Figures


References
-
- Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian cancer statistics 2015. Toronto (ON): Canadian Cancer Society; 2015.
-
- Prostate cancer [Internet]. Toronto (ON): Canadian Cancer Society; c2016. [updated 2015; cited 2016 Feb 8]. Available from: http://www.cancer.ca/en/cancer-information/cancer-type/prostate/prostate...
-
- Cancer Care Ontario. Ontario cancer statistics 2016 [Internet]. Toronto (ON): Cancer Care Ontario; 2016. Available from: https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=360956
-
- Sun F, Oyesanmi O, Fontanarosa J, Reston J, Guzzo T, Schoelles K. Therapies for clinically localized prostate cancer: update of a 2008 systematic review. Rockville (MD): Agency for Healthcare Research and Quality; 2014. - PubMed
-
- Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, et al. NIH State-of-the-Science Conference Statement: role of active surveillance in the management of men with localized prostate cancer. NIH Consens State Sci Statements. 2011; 28 (1): 1–27. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
