Effects of ligand distribution on receptor-diffusion-mediated cellular uptake of nanoparticles
- PMID: 28573012
- PMCID: PMC5451813
- DOI: 10.1098/rsos.170063
Effects of ligand distribution on receptor-diffusion-mediated cellular uptake of nanoparticles
Abstract
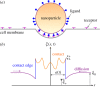
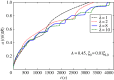
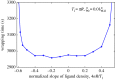
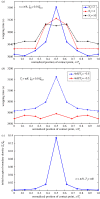
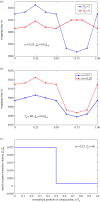
Biophysical-factor-dependent cellular uptake of nanoparticles (NPs) through receptor-diffusion-mediated endocytosis bears significance in pathology, cellular immunity and drug-delivery systems. Advanced nanotechnology of NP synthesis provides methods for modifying NP surface with different ligand distributions. However, no report discusses effects of ligand distribution on NP surface on receptor-diffusion-mediated cellular uptake. In this article, we used a statistical dynamics model of receptor-diffusion-mediated endocytosis to examine ligand-distribution-dependent cellular uptake dynamics by considering that ligand-receptor complexes drive engulfing to overcome resistance to membrane deformation and changes in configuration entropy of receptors. Results showed that cellular internalization of NPs strongly depended on ligand distribution and that cellular-uptake efficiency of NPs was high when ligand distribution was within a range around uniform distribution. This feature of endocytosis ensures robust infection ability of viruses to enter host cells. Interestingly, results also indicated that optimal ligand distribution associated with highest cellular-uptake efficiency slightly depends on distribution pattern of ligands and density of receptors, and the optimal distribution becomes uniform when receptor density is sufficiently large. Position of initial contact point is also a factor affecting dynamic wrapping. This study explains why most enveloped viruses present almost homogeneous ligand distribution and is useful in designing controlled-release drug-delivery systems.
Keywords: cellular uptake; controlled-release drug delivery; optimal ligand distribution; receptor diffusion.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hillaireau H, Couvreur P. 2009. Nanocarriers' entry into the cell: relevance to drug delivery. Cell. Mol. Life Sci. 66, 2873–2896. (doi:10.1007/s00018-009-0053-z) - DOI - PMC - PubMed
-
- Reynwar BJ, Illya G, Harmandaris VA, Müller MM, Kremer K, Deserno M. 2007. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 447, 461–464. (doi:10.1038/nature05840) - DOI - PubMed
-
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. 2009. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557. (doi:10.1038/nmat2442) - DOI - PubMed
-
- Poland CA, et al. 2008. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 3, 423–428. (doi:10.1038/nnano.2008.111) - DOI - PubMed
-
- Sahay G, Alakhova DY, Kabanov AV. 2010. Endocytosis of nanomedicines. J. Control. Release 145, 182–195. (doi:10.1016/j.jconrel.2010.01.036) - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
