Modeling the Effect of the Aryl Hydrocarbon Receptor on Transplant Immunity
- PMID: 28573192
- PMCID: PMC5441988
- DOI: 10.1097/TXD.0000000000000666
Modeling the Effect of the Aryl Hydrocarbon Receptor on Transplant Immunity
Abstract
Background: Exposure to pollutants through inhalation is a risk factor for lung diseases including cancer, asthma, and lung transplant rejection, but knowledge of the effects of inhaled pollutants on pathologies outside of the lung is limited.
Methods: Using the minor-mismatched model of male C57BL/6J (B6) to female B6 skin grafts, recipient mice were treated with an inhaled urban dust particle sample every 3 days before and after grafting. Graft survival time was determined, and analysis of the resulting immune response was performed at time before rejection.
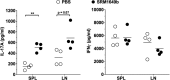
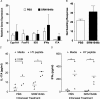
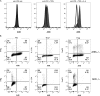
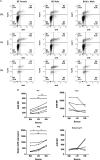
Results: Significant prolongation of male skin grafts occurred in recipient female mice treated with urban dust particles compared with controls and was found to be dependent on aryl hydrocarbon receptor (AHR) expression in the recipient mouse. T cell responses to the male histocompatibility antigen (H-Y) Dby were not altered by exposure to pollutants. A reduction in the frequency of IFNγ-producing CD4 T cells infiltrating the graft on day 7 posttransplant was observed. Flow cytometry analysis revealed that AHR expression is upregulated in IFNγ-producing CD4 T cells during immune responses in vitro and in vivo.
Conclusions: Surprisingly, inhalation of a pollutant standard was found to prolong graft survival in a minor-mismatched skin graft model in an AHR-dependent manner. One possible mechanism may be an effect on IFNγ-producing CD4 T cells responding to donor antigen. The increased expression of AHR in this CD4 T cell subset suggests that AHR ligands within the particulate matter may be directly affecting the type 1 T helper cell response in this model.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. - PubMed
-
- Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. - PubMed
-
- Quintana FJ, Basso AS, Iglesias AH, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

