Prediction of Organic Reaction Outcomes Using Machine Learning
- PMID: 28573205
- PMCID: PMC5445544
- DOI: 10.1021/acscentsci.7b00064
Prediction of Organic Reaction Outcomes Using Machine Learning
Abstract
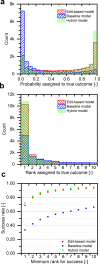
Computer assistance in synthesis design has existed for over 40 years, yet retrosynthesis planning software has struggled to achieve widespread adoption. One critical challenge in developing high-quality pathway suggestions is that proposed reaction steps often fail when attempted in the laboratory, despite initially seeming viable. The true measure of success for any synthesis program is whether the predicted outcome matches what is observed experimentally. We report a model framework for anticipating reaction outcomes that combines the traditional use of reaction templates with the flexibility in pattern recognition afforded by neural networks. Using 15 000 experimental reaction records from granted United States patents, a model is trained to select the major (recorded) product by ranking a self-generated list of candidates where one candidate is known to be the major product. Candidate reactions are represented using a unique edit-based representation that emphasizes the fundamental transformation from reactants to products, rather than the constituent molecules' overall structures. In a 5-fold cross-validation, the trained model assigns the major product rank 1 in 71.8% of cases, rank ≤3 in 86.7% of cases, and rank ≤5 in 90.8% of cases.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Corey E. J. General methods for the construction of complex molecules. Pure Appl. Chem. 1967, 14, 19–38. 10.1016/B978-0-08-020741-4.50004-X. - DOI
-
- Pensak D. A.; Corey E. J.. Computer-Assisted Organic Synthesis; ACS Symp. Ser.; 1977; Vol. 61; Chapter 1, pp 1–32, doi: 10.1021/bk-1977-0061.ch001. - DOI
-
- Satoh H.; Funatsu K. SOPHIA, a Knowledge Base-Guided Reaction Prediction System - Utilization of a Knowledge Base Derived from a Reaction Database. J. Chem. Inf. Model. 1995, 35, 34–44. 10.1021/ci00023a005. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

