Projected long-term outcomes in patients with type 1 diabetes treated with fast-acting insulin aspart vs conventional insulin aspart in the UK setting
- PMID: 28573681
- PMCID: PMC5697732
- DOI: 10.1111/dom.13026
Projected long-term outcomes in patients with type 1 diabetes treated with fast-acting insulin aspart vs conventional insulin aspart in the UK setting
Abstract
Aim: To assess the impact of faster aspart vs insulin aspart on long-term clinical outcomes and costs for patients with type 1 diabetes mellitus (T1DM) in the UK setting.
Methods: The QuintilesIMS CORE Diabetes Model was used to project clinical outcomes and costs over patient lifetimes in a cohort with data on baseline characteristics from the "onset 1" trial. Treatment effects were taken from the 26-week main phase of the onset 1 trial, with costs and utilities based on literature review. Future costs and clinical benefits were discounted at 3.5% annually.
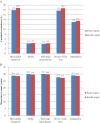
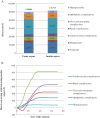
Results: Projections indicated that faster aspart was associated with improved discounted quality-adjusted life expectancy (by 0.13 quality-adjusted life-years) vs insulin aspart. Improved clinical outcomes resulted from fewer diabetes-related complications and a delayed time to their onset with faster aspart. Faster aspart was found to be associated with reduced costs vs insulin aspart (cost savings of £1715), resulting from diabetes-related complications avoided and reduced treatment costs.
Conclusions: Faster aspart was associated with improved clinical outcomes and cost savings vs insulin aspart for patients with T1DM in the UK setting.
Keywords: cost-effectiveness; insulin therapy; type 1 diabetes.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
B. H. and W. J. V. are employees of Ossian Health Economics and Communications. Ossian received funding from Novo Nordisk A/S to perform the present analysis. S. B. was an employee of Novo Nordisk when this research was conducted. A. S. is an employee of Novo Nordisk A/S. D. R.‐J. has received research funding, advisory panel fees and lecture panel honoraria from Astra Zenica, Boehringer Ingelheim, Cellnovo, Lilly, Novartis, Novo Nordisk, and Sanofi. S. R. H. has received personal fees from Sanofi Aventis, Eli Lilly, Takeda, Novo Nordisk and Astra Zeneca for serving on Speaker panels, and is an employee of the University of Sheffield, which has received remuneration from Eli Lilly, Boeringher Ingelheim, Novo Nordisk, and Takeda for consultancy.
Figures
References
-
- National Institute for Health and Care Excellence . NG17: Type 1 diabetes in adults: diagnosis and management. 2015. Available at: http://www.nice.org.uk/guidance/ng17. Accessed November 1, 2016. - PubMed
-
- National Institute for Health and Care Excellence . NG18: Diabetes (type 1 and type 2) in children and young people: diagnosis and management. 2015. Available at: http://www.nice.org.uk/guidance/ng18. Accessed November 1, 2016. - PubMed
-
- National Diabetes Audit 2011–2012. Report 2: Complications and Mortality. 2013. Available at: http://www.hscic.gov.uk/catalogue/PUB12738/nati‐diab‐audi‐11‐12‐mort‐com.... Accessed November 1, 2016.
-
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29(7):855–862. - PubMed
-
- The DCCT Research Group , Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

