Isolation, Amino Acid Sequences, and Plausible Functions of the Galacturonic Acid-Binding Egg Lectin of the Sea Hare Aplysia kurodai
- PMID: 28574432
- PMCID: PMC5484111
- DOI: 10.3390/md15060161
Isolation, Amino Acid Sequences, and Plausible Functions of the Galacturonic Acid-Binding Egg Lectin of the Sea Hare Aplysia kurodai
Abstract
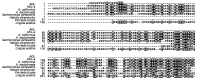
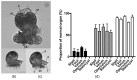
Egg lectins occur in a variety of animals ranging from mollusks to vertebrates. A few examples of molluscan egg lectins have been reported, including that of the sea hare Aplysia kurodai; however, their biological functions in the egg remain unclarified. We report the isolation, determination of primary structure, and possible functions of A.kurodai lectin (AKL) from the egg mass of A. kurodai. We obtained AKL as an inseparable mixture of isoproteins with a relative molecular mass of approximately 32 kDa by affinity purification. The hemagglutinating activity of AKL against rabbit erythrocytes was inhibited most potently by galacturonic acid and moderately by xylose. Nucleotide sequencing of corresponding cDNA obtained by rapid amplification of cDNA ends (RACE) allowed us to deduce complete amino acid sequences. The mature polypeptides consisted of 218- or 219-amino acids with three repeated domains. The amino acid sequence had similarities to hypothetical proteins of Aplysia spp., or domain DUF3011 of uncharacterized bacterial proteins. AKL is the first member of the DUF3011 family whose function, carbohydrate recognition, was revealed. Treatment of the egg with galacturonic acid, an AKL sugar inhibitor, resulted in deformation of the veliger larvae, suggesting that AKL is involved in organogenesis in the developmental stage of A. kurodai.
Keywords: Aplysia kurodai; egg lectin; embryonic development; galacturonic acid.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures




References
-
- Jimbo M., Yamashita H., Koike K., Sakai R., Kamiya H. Effects of lectin in the scleractinian coral Ctenactis echinata on symbiotic zooxanthellae. Fish. Sci. 2010;76:355–363. doi: 10.1007/s12562-009-0204-z. - DOI
-
- Ueda T., Nakamura Y., Smith C.M., Copits B.A., Inoue A., Ojima T., Matsunaga S., Swanson G.T., Sakai R. Isolation of novel prototype galectins from the marine ball sponge Cinachyrella sp. guided by their modulatory activity on mammalian glutamate-gated ion channels. Glycobiology. 2013;23:412–425. doi: 10.1093/glycob/cws165. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

