Pathogen-induced ubiquitin-editing enzyme A20 bifunctionally shuts off NF-κB and caspase-8-dependent apoptotic cell death
- PMID: 28574503
- PMCID: PMC5563994
- DOI: 10.1038/cdd.2017.89
Pathogen-induced ubiquitin-editing enzyme A20 bifunctionally shuts off NF-κB and caspase-8-dependent apoptotic cell death
Abstract
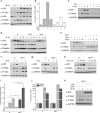
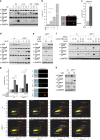
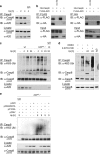
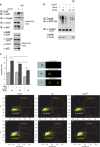
The human pathogen Helicobacter pylori infects more than half of the world's population and is a paradigm for persistent yet asymptomatic infection but increases the risk for chronic gastritis and gastric adenocarcinoma. For successful colonization, H. pylori needs to subvert the host cell death response, which serves to confine pathogen infection by killing infected cells and preventing malignant transformation. Infection of gastric epithelial cells by H. pylori provokes direct and fast activation of the proinflammatory and survival factor NF-κB, which regulates target genes, such as CXCL8, BIRC3 and TNFAIP3. However, it is not known how H. pylori exploits NF-κB activation and suppresses the inflammatory response and host apoptotic cell death, in order to avert the innate immune response and avoid cell loss, and thereby enhance colonization to establish long-term infection. Here we assign for the first time that H. pylori and also Campylobacter jejuni-induced ubiquitin-editing enzyme A20 bifunctionally terminates NF-κB activity and negatively regulates apoptotic cell death. Mechanistically, we show that the deubiquitinylase activity of A20 counteracts cullin3-mediated K63-linked ubiquitinylation of procaspase-8, therefore restricting the activity of caspase-8. Interestingly, another inducible NF-κB target gene, the scaffold protein p62, ameliorates the interaction of A20 with procaspase-8. In conclusion, pathogen-induced de novo synthesis of A20 regulates the shut-off of the survival factor NF-κB but, on the other hand, also impedes caspase-8-dependent apoptotic cell death so as to promote the persistence of pathogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A20 undermines alternative NF-κB activity and expression of anti-apoptotic genes in Helicobacter pylori infection.Cell Mol Life Sci. 2022 Jan 28;79(2):102. doi: 10.1007/s00018-022-04139-y. Cell Mol Life Sci. 2022. PMID: 35089437 Free PMC article.
-
CYLD-TRAF6 interaction promotes ADP-heptose-induced NF-κB signaling in H. pylori infection.EMBO Rep. 2025 Jul;26(13):3241-3263. doi: 10.1038/s44319-025-00480-y. Epub 2025 May 22. EMBO Rep. 2025. PMID: 40404856 Free PMC article.
-
A20 negatively regulates T cell receptor signaling to NF-kappaB by cleaving Malt1 ubiquitin chains.J Immunol. 2009 Jun 15;182(12):7718-28. doi: 10.4049/jimmunol.0803313. J Immunol. 2009. PMID: 19494296
-
ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling.Biochem Pharmacol. 2009 Jul 15;78(2):105-14. doi: 10.1016/j.bcp.2009.02.009. Epub 2009 Feb 27. Biochem Pharmacol. 2009. PMID: 19464428 Review.
-
The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology.Trends Immunol. 2009 Aug;30(8):383-91. doi: 10.1016/j.it.2009.05.007. Epub 2009 Jul 28. Trends Immunol. 2009. PMID: 19643665 Review.
Cited by
-
NF-κB-regulated ubiquitin-editing enzyme A20 paves the way for infection persistency.Cell Cycle. 2018;17(1):3-4. doi: 10.1080/15384101.2017.1387435. Epub 2018 Jan 4. Cell Cycle. 2018. PMID: 29099284 Free PMC article. No abstract available.
-
A20 Restricts Inflammatory Response and Desensitizes Gingival Keratinocytes to Apoptosis.Front Immunol. 2020 Mar 10;11:365. doi: 10.3389/fimmu.2020.00365. eCollection 2020. Front Immunol. 2020. PMID: 32218782 Free PMC article.
-
The involvement of regulated cell death forms in modulating the bacterial and viral pathogenesis.Int Rev Cell Mol Biol. 2020;353:211-253. doi: 10.1016/bs.ircmb.2019.12.008. Epub 2020 Jan 27. Int Rev Cell Mol Biol. 2020. PMID: 32381176 Free PMC article. Review.
-
Manifold role of ubiquitin in Helicobacter pylori infection and gastric cancer.Cell Mol Life Sci. 2021 May;78(10):4765-4783. doi: 10.1007/s00018-021-03816-8. Epub 2021 Apr 7. Cell Mol Life Sci. 2021. PMID: 33825941 Free PMC article. Review.
-
Research Progress for Targeting Deubiquitinases in Gastric Cancers.Cancers (Basel). 2022 Nov 26;14(23):5831. doi: 10.3390/cancers14235831. Cancers (Basel). 2022. PMID: 36497313 Free PMC article. Review.
References
-
- Shanks AM, El-Omar EM. Helicobacter pylori infection, host genetics and gastric cancer. J Dig Dis 2009; 10: 157–164. - PubMed
-
- Backert S, Clyne M. Pathogenesis of Helicobacter pylori infection. Helicobacter 2011; 16(Suppl 1): 19–25. - PubMed
-
- Sokolova O, Maubach G, Naumann M. MEKK3 and TAK1 synergize to activate IKK complex in Helicobacter pylori infection. Biochim Biophys Acta 2014; 1843: 715–724. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

