NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy
- PMID: 28574504
- PMCID: PMC5563995
- DOI: 10.1038/cdd.2017.91
NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy
Abstract
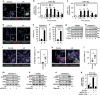
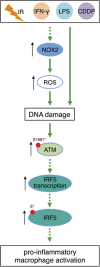
Although tumor-associated macrophages have been extensively studied in the control of response to radiotherapy, the molecular mechanisms involved in the ionizing radiation-mediated activation of macrophages remain elusive. Here we show that ionizing radiation induces the expression of interferon regulatory factor 5 (IRF5) promoting thus macrophage activation toward a pro-inflammatory phenotype. We reveal that the activation of the ataxia telangiectasia mutated (ATM) kinase is required for ionizing radiation-elicited macrophage activation, but also for macrophage reprogramming after treatments with γ-interferon, lipopolysaccharide or chemotherapeutic agent (such as cisplatin), underscoring the fact that the kinase ATM plays a central role during macrophage phenotypic switching toward a pro-inflammatory phenotype through the regulation of mRNA level and post-translational modifications of IRF5. We further demonstrate that NADPH oxidase 2 (NOX2)-dependent ROS production is upstream to ATM activation and is essential during this process. We also report that the inhibition of any component of this signaling pathway (NOX2, ROS and ATM) impairs pro-inflammatory activation of macrophages and predicts a poor tumor response to preoperative radiotherapy in locally advanced rectal cancer. Altogether, our results identify a novel signaling pathway involved in macrophage activation that may enhance the effectiveness of radiotherapy through the reprogramming of tumor-infiltrating macrophages.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Down-regulation of NOX2 activity in phagocytes mediated by ATM-kinase dependent phosphorylation.Free Radic Biol Med. 2017 Dec;113:1-15. doi: 10.1016/j.freeradbiomed.2017.09.007. Epub 2017 Sep 13. Free Radic Biol Med. 2017. PMID: 28916473 Free PMC article.
-
Investigation of switch from ATM to ATR signaling at the sites of DNA damage induced by low and high LET radiation.DNA Repair (Amst). 2013 Dec;12(12):1143-51. doi: 10.1016/j.dnarep.2013.10.004. Epub 2013 Nov 12. DNA Repair (Amst). 2013. PMID: 24238855
-
Tumor suppressor ataxia telangiectasia mutated functions downstream of TGF-β1 in orchestrating profibrotic responses.FASEB J. 2015 Apr;29(4):1258-68. doi: 10.1096/fj.14-262527. Epub 2014 Dec 5. FASEB J. 2015. PMID: 25480384 Free PMC article.
-
Mechanisms of ATM Activation.Annu Rev Biochem. 2015;84:711-38. doi: 10.1146/annurev-biochem-060614-034335. Epub 2015 Jan 12. Annu Rev Biochem. 2015. PMID: 25580527 Review.
-
Neurodegeneration in ataxia-telangiectasia: Multiple roles of ATM kinase in cellular homeostasis.Dev Dyn. 2018 Jan;247(1):33-46. doi: 10.1002/dvdy.24522. Epub 2017 Jun 5. Dev Dyn. 2018. PMID: 28543935 Review.
Cited by
-
Radiation-Induced Immunity and Toxicities: The Versatility of the cGAS-STING Pathway.Front Immunol. 2021 May 17;12:680503. doi: 10.3389/fimmu.2021.680503. eCollection 2021. Front Immunol. 2021. PMID: 34079557 Free PMC article. Review.
-
Dual oxidase 1 limits the IFNγ-associated antitumor effect of macrophages.J Immunother Cancer. 2020 Jun;8(1):e000622. doi: 10.1136/jitc-2020-000622. J Immunother Cancer. 2020. PMID: 32571996 Free PMC article.
-
Wound Healing versus Metastasis: Role of Oxidative Stress.Biomedicines. 2022 Nov 2;10(11):2784. doi: 10.3390/biomedicines10112784. Biomedicines. 2022. PMID: 36359304 Free PMC article. Review.
-
Macrophages in radiation injury: a new therapeutic target.Oncoimmunology. 2018 Jul 23;7(10):e1494488. doi: 10.1080/2162402X.2018.1494488. eCollection 2018. Oncoimmunology. 2018. PMID: 30288363 Free PMC article.
-
Identification and validation of biomarkers related to ferroptosis in idiopathic pulmonary fibrosis.Sci Rep. 2025 Mar 13;15(1):8622. doi: 10.1038/s41598-025-93217-9. Sci Rep. 2025. PMID: 40075162 Free PMC article.
References
-
- Hekim N, Cetin Z, Nikitaki Z, Cort A, Saygili EI. Radiation triggering immune response and inflammation. Cancer Lett 2015; 368: 156–163. - PubMed
-
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31: 51–72. - PubMed
-
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15: 1170–1178. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

