Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice
- PMID: 28574506
- PMCID: PMC5563990
- DOI: 10.1038/cdd.2017.84
Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice
Abstract
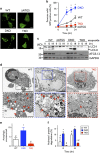
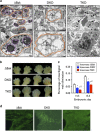
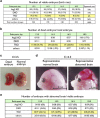
Programmed cell death, which is required for the development and homeostasis of metazoans, includes mechanisms such as apoptosis, autophagic cell death, and necrotic (or type III) death. Members of the Bcl2 family regulate apoptosis, among which Bax and Bak act as a mitochondrial gateway. Although embryonic fibroblasts from Bax/Bak double-knockout (DKO) mice are resistant to apoptosis, we previously demonstrated that these cells die through an autophagy-dependent mechanism in response to various types of cellular stressors. To determine the physiological role of autophagy-dependent cell death, we generated Atg5/Bax/Bak triple-knockout (TKO) mice, in which autophagy is greatly suppressed compared with DKO mice. Embryonic fibroblasts and thymocytes from TKO mice underwent autophagy much less frequently, and their viability was much higher than DKO cells in the presence of certain cellular stressors, providing genetic evidence that DKO cells undergo Atg5-dependent death. Compared with wild-type embryos, the loss of interdigital webs was significantly delayed in DKO embryos and was even further delayed in TKO embryos. Brain malformation is a distinct feature observed in DKO embryos on the 129 genetic background, but not in those on a B6 background, whereas such malformations appeared in TKO embryos even on a B6 background. Taken together, our data suggest that Atg5-dependent cell death contributes to the embryonic development of DKO mice, implying that autophagy compensates for the deficiency in apoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol 1990; 181: 195–213. - PubMed
-
- Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 2003; 195: 158–167. - PubMed
-
- Baehrecke EH. How death shapes life during development. Nat Rev Mol Cell Biol 2002; 3: 779–787. - PubMed
-
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 2007; 9: 1102–1109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

