Neuroprotective effects respond to cerebral ischemia without susceptibility to HB-tumorigenesis in VHL heterozygous knockout mice
- PMID: 28574654
- PMCID: PMC5583010
- DOI: 10.1002/mc.22688
Neuroprotective effects respond to cerebral ischemia without susceptibility to HB-tumorigenesis in VHL heterozygous knockout mice
Abstract
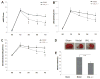
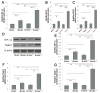
The von Hippel-Lindau (VHL) tumor suppressor gene plays a prominent role in the development of hemangioblastomas (HBs) within specific regions of the human' central nervous system (CNS). Alterations in VHL gene are rarely observed in the more common features of human VHL-related tumors in animal models, and VHL heterozygous knockout (VHL+/-) mice do not develop HBs. We tested whether VHL heterozygous knockout mice exhibited genetic predisposition to the development of HBs and conferred a selective advantage involving growth of blood vessels to its carrier. No differences were observed between wild-type and VHL+/- mice in development ad reproduction. The heterozygous VHL+/- mice did not develop higher genetic susceptibility to CNS-HBs over their lifetime. Furthermore, this recessive VHL gene heterozygosity is relatively stable. Interestingly, we found these heterozygous VHL+/- mice gained an advantage conferring to angiogenic ability in a particular environment, compared with wild-type mice. The heterozygous VHL+/- mice obviously enhanced hypoxia inducible factor-1 (HIF)-dependent and Twist1 angiogenic mechanism in response to acute cerebral ischemia, resulting in decreased cerebral tissue damage and neuroprotective response through neovascularization. Our findings provide evidence of partial loss function of VHL as a novel precise therapeutic target in acute cerebral ischemia.
Keywords: cerebral ischaemia; gene therapy; hemangioblastoma; neuroprotection; von Hippel-Lindau gene (VHL).
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Figures


Similar articles
-
Endothelial cells by inactivation of VHL gene direct angiogenesis, not vasculogenesis via Twist1 accumulation associated with hemangioblastoma neovascularization.Sci Rep. 2017 Jul 14;7(1):5463. doi: 10.1038/s41598-017-05833-9. Sci Rep. 2017. PMID: 28710479 Free PMC article.
-
Analysis of von hippel-lindau mutations with comparative genomic hybridization in sporadic and hereditary hemangioblastomas: possible genetic heterogeneity.J Neurosurg. 2002 Oct;97(4):977-82. doi: 10.3171/jns.2002.97.4.0977. J Neurosurg. 2002. PMID: 12405390
-
Loss of heterozygosity and somatic mutations of the VHL tumor suppressor gene in sporadic cerebellar hemangioblastomas.Cancer Res. 1998 Feb 1;58(3):504-8. Cancer Res. 1998. PMID: 9458097
-
Von hippel-lindau disease: a genetic and clinical review.Semin Ophthalmol. 2013 Sep-Nov;28(5-6):377-86. doi: 10.3109/08820538.2013.825281. Semin Ophthalmol. 2013. PMID: 24138046 Review.
-
Pathology, genetics and cell biology of hemangioblastomas.Histol Histopathol. 1996 Oct;11(4):1049-61. Histol Histopathol. 1996. PMID: 8930647 Review.
Cited by
-
A Comprehensive Procedure to Evaluate the In Vitro Performance of the Putative Hemangioblastoma Neovascularization Using the Spheroid Sprouting Assay.J Vis Exp. 2018 Apr 12;(134):57183. doi: 10.3791/57183. J Vis Exp. 2018. PMID: 29708531 Free PMC article.
References
-
- Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–20. - PubMed
-
- van Rooijen E, Voest EE, Logister I, et al. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Disease models & mechanisms. 2010;3(5–6):343–53. doi: 10.1242/dmm.004036. [published Online First: Epub Date]|. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

